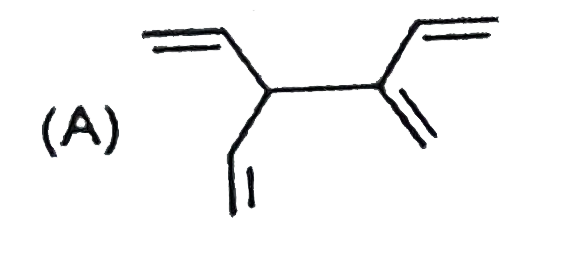
A
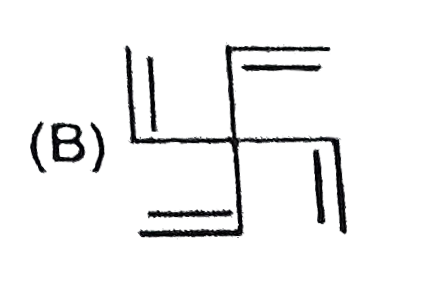
B
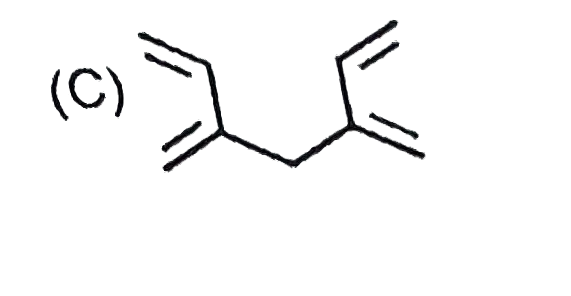
C
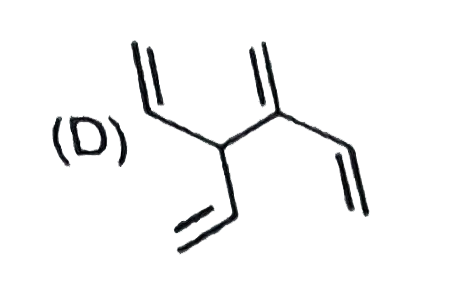
D
Text Solution
AI Generated Solution
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-DPP-QUESTIONS
- The IUPAC name for the following compound is
Text Solution
|
- Consider the following compound: The IUPAC name of the this compo...
Text Solution
|
- Diethenyl pentadiene is :
Text Solution
|
- IUPAC name of the following compound will be
Text Solution
|
- Which of the following functional group is non chain terminating funct...
Text Solution
|
- Which of the following compound is cumulative diene
Text Solution
|
- Which of the following compound have conjugated diene
Text Solution
|
- Which of the following compound does not have isolated diene
Text Solution
|
- Which of the following has correct IUPAC numbering
Text Solution
|
- Which is /are secondary alcohol
Text Solution
|
- Which of the following is/are cresol
Text Solution
|
- Which of the following is/are benzyl derivative :
Text Solution
|
- Which the sum of position of functional group and substituent in the g...
Text Solution
|
- In how many of the following compound. -OH group directly attached on ...
Text Solution
|
- How many of the following are tertiary amines ?
Text Solution
|
- In the compound H3C-undersetunderset(OH)(|)CH-CH2-CH2-undersetunderset...
Text Solution
|
- How many ketones of the following has hex root word ?
Text Solution
|
- Match the column :
Text Solution
|
- Correct IUPAC name of the following compound is :
Text Solution
|
- Correct IUPAC name of the following compound is :
Text Solution
|