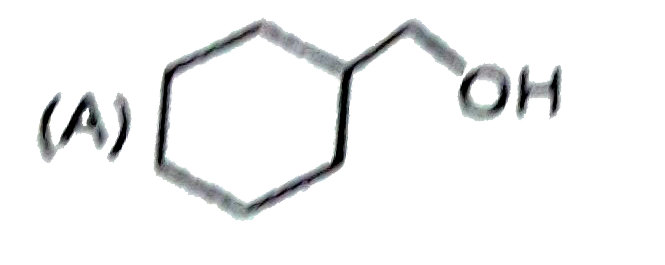
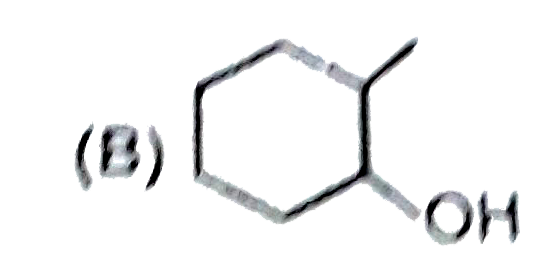
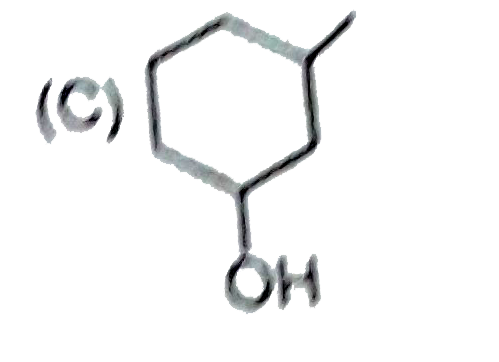
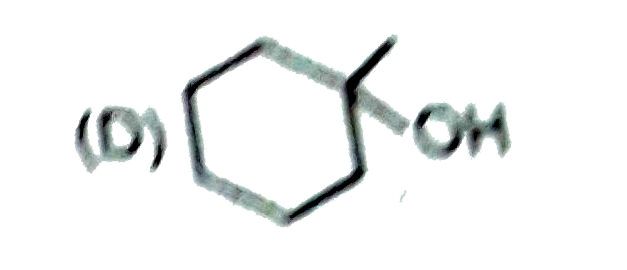
A
B
C
D
Text Solution
Verified by Experts
RESONANCE ENGLISH-DPP-QUESTIONS
- CH3-undersetunderset(OH)(|)oversetoverset(CH3)(|)C-CH3 undersetDeltaov...
Text Solution
|
- Ph-CH=CH2+HBr "Peroxide"toMajor product is :
Text Solution
|
- Complete the following reaction
Text Solution
|
- A compound (C5H8) gives with ppt. with ammonical AgNO3 and on reductio...
Text Solution
|
- CH3-CH2-CH2-CHCl2 undersetDeltaoverset(NaNH2)to Major product is :
Text Solution
|
- CH3-C-=C-CH3+HBr to Major product is :
Text Solution
|
- The major product of the following reaction is: CH(3)C-=CH underset((...
Text Solution
|
- Ph-C-=Chunderset(H2O2//OH^-)overset(B2H6)to Major Product is :
Text Solution
|
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|
- CaC2 overset(H2O)to AB overset(Hg^(+2)//H2SO4)to What is B ?
Text Solution
|
- A compound (X) with molecular C6H10 which shows Baeyer's test. Compoun...
Text Solution
|
- A compound (A) with molecular formula C4H8O shows bromine water test. ...
Text Solution
|
- Which of the following functional groups is identied by Baeyer's test ...
Text Solution
|
- Camphene is constituent of pine oil. It has following structure. ...
Text Solution
|
- Complete the following reaction
Text Solution
|
- CH3CH2C-=C CH2CH3+H2O overset(HgSO4)to Product, Product is :
Text Solution
|
- Complete the following reaction
Text Solution
|
- Treatment of phenol with bromine dissolved in C Cl4 gives :
Text Solution
|
- The e.m.f of cell Ag|AgI((s)),0.05M KI|| 0.05 M AgNO(3)|Ag is 0.788 V....
Text Solution
|