A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
QUALITATIVE ANALYSIS (ANION)
RESONANCE ENGLISH|Exercise Exercise-2|44 VideosQUALITATIVE ANALYSIS (ANION)
RESONANCE ENGLISH|Exercise Exercise-3|10 VideosQUALITATIVE ANALYSIS (ANION)
RESONANCE ENGLISH|Exercise Matching List Type|1 VideosQUALITATIVE ANALYSIS
RESONANCE ENGLISH|Exercise INORGANIC CHMISTRY(Qualitative analysis)|35 VideosQUALITATIVE ANALYSIS PART 1
RESONANCE ENGLISH|Exercise A.L.P|39 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-QUALITATIVE ANALYSIS (ANION)-Exercise-1
- Gas that turns lime water milky and aciddied K(2)Cr(2)O(7) green is...
Text Solution
|
- A gas which turns lead acetate paper black.
Text Solution
|
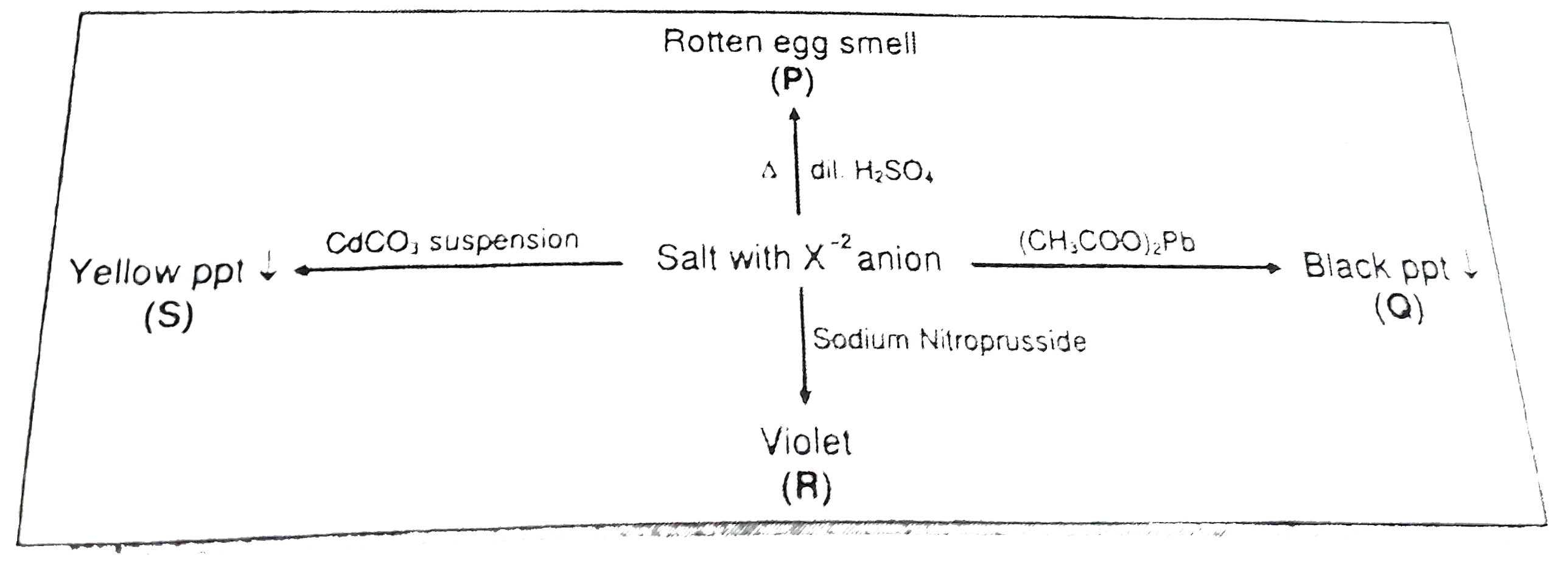
- Anion (X^(2-)) is :
Text Solution
|
- At freezing point of a solution there is always
Text Solution
|
- Sulphide ion reacts with [Fe(CN)(5)NO] to form a purple coloured compo...
Text Solution
|
- Which of the following pair of anions are identified by conc. H(2)SO(4...
Text Solution
|
- Which of the following anion behaves in a different manner than other ...
Text Solution
|
- Which of the following reagents turns white precipitate of AgCl yellow...
Text Solution
|
- A mixture when heated with dil H(2)SO(4) does not evolve brown vapour...
Text Solution
|
- When a mixture of solid NaCl and solid K(2)Cr(2)O(7) is heated with co...
Text Solution
|
- AgCl dissolves in ammonia solution giving:
Text Solution
|
- A mixture upon along adding conc. H(2)SO(4) gives deep red fumes. It m...
Text Solution
|
- The acidic solution of a salt produces blue colour with Kl starch solu...
Text Solution
|
- A colourless solution of a compound gives a precipitate with AgNO(3) s...
Text Solution
|
- Which of the following gas turn starch iodide paper blue ?
Text Solution
|
- Nitrates is confirmed by ring test.The brown colour of the ring is due...
Text Solution
|
- When a saturated solution of sodium chloride is heated it
Text Solution
|
- A metal salt solution gives a yellow precipitate with silver nitrate.T...
Text Solution
|
- Match the anions with the changes observed on qualitative analysis :
Text Solution
|
- Match the reagent which are used in qualitative analysis of given anio...
Text Solution
|