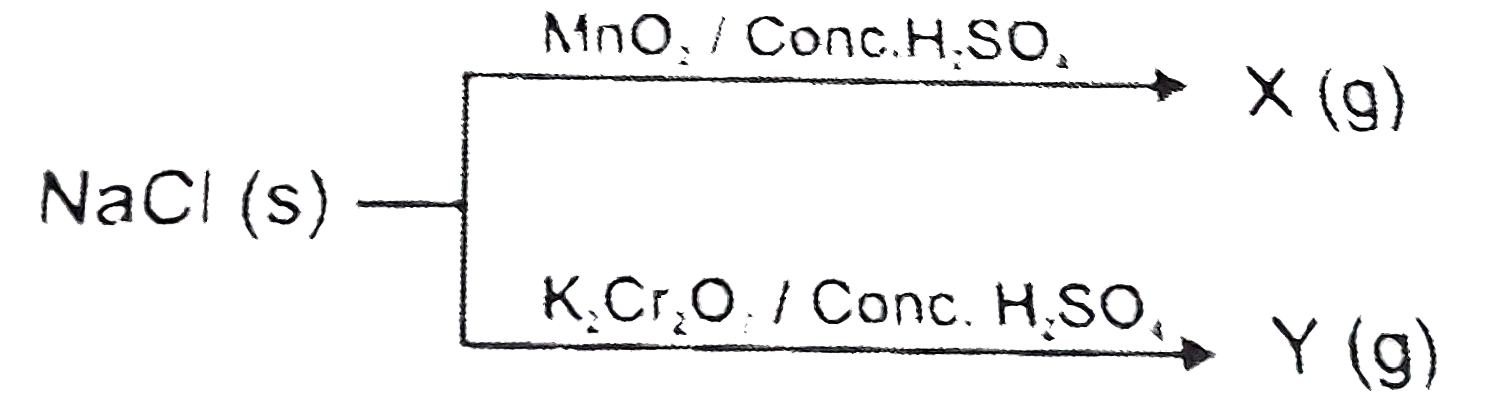Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
QUALITATIVE ANALYSIS (ANION)
RESONANCE ENGLISH|Exercise Exercise-3|10 VideosQUALITATIVE ANALYSIS (ANION)
RESONANCE ENGLISH|Exercise Additional Problems for Self Practice (APSP)|30 VideosQUALITATIVE ANALYSIS (ANION)
RESONANCE ENGLISH|Exercise Exercise-1|59 VideosQUALITATIVE ANALYSIS
RESONANCE ENGLISH|Exercise INORGANIC CHMISTRY(Qualitative analysis)|35 VideosQUALITATIVE ANALYSIS PART 1
RESONANCE ENGLISH|Exercise A.L.P|39 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-QUALITATIVE ANALYSIS (ANION)-Exercise-2
- Na(2)SO(3), NaCl, Na(2)C(2)O(4),Na(2)CrO(4),NaNO(2),CH(3)CO(2)Na are s...
Text Solution
|
- B(3)^(3-)+Conc.H(2)SO(4)+CH(3)-CH(2)-OH overset(ignite)rarr(A) What ...
Text Solution
|
- a=difference in the oxidation number of Cl in the product X and produc...
Text Solution
|
- Which of the following salt liberates a colourless gas on acidificatio...
Text Solution
|
- Which of the following salts release reddish brown gas when heated in ...
Text Solution
|
- Which of the following can decompose on heating to give CO(2) ?
Text Solution
|
- Metals which do not give flame test ?
Text Solution
|
- In the following diagram bunsen flame the (X ) represent.
Text Solution
|
- Which of the following respond to borax test ?
Text Solution
|
- This gas turns lime water milky.
Text Solution
|
- Then A may have :
Text Solution
|
- S^(2-) " and" SO(3)^(2-) can be distinguished by :
Text Solution
|
- Which statements is/are correct about sodium nitroprusside test ?
Text Solution
|
- Which statement(s) is /are correct about Brown ring test ?
Text Solution
|
- Which of the following metal chlordie will give chromyl chloride test ...
Text Solution
|
- Which of the following will be completely or parially dissolved in NH...
Text Solution
|
- Reddish brown gas is obtained when the following are treated with conc...
Text Solution
|
- Each of these solution is added to a mixture of aqueous solution oo...
Text Solution
|
- underset("(mixture of two anions)")A underset("excess of "BaCl2)overse...
Text Solution
|
- Which among the following chloride salts does not form HCl as one of t...
Text Solution
|