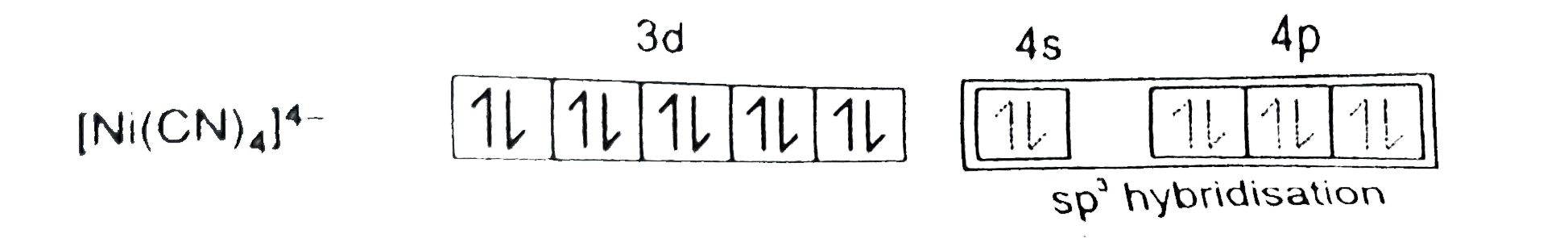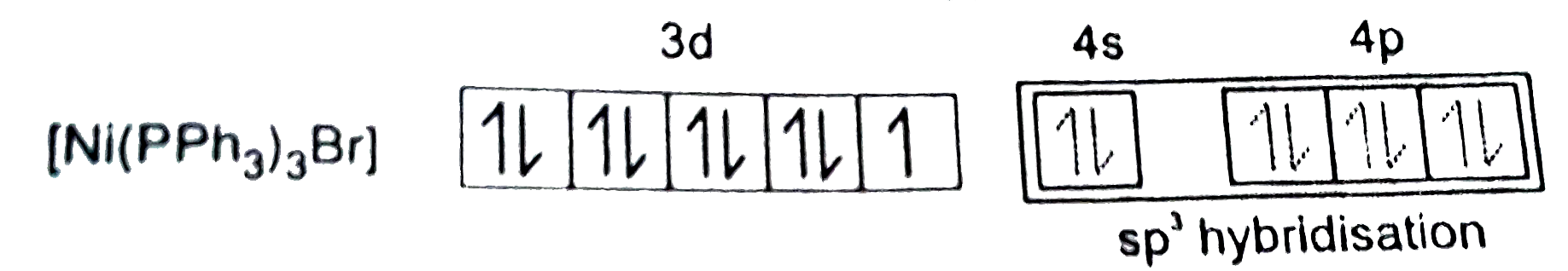A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
RESONANCE ENGLISH|Exercise Exercise Part-I: Subjective Question Section (A)|5 VideosCOORDINATION COMPOUNDS
RESONANCE ENGLISH|Exercise Exercise Part-I: Subjective Question Section (B)|4 VideosCOORDINATION COMPOUNDS
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Hydrocarbon)|7 VideosCHEMISTRY IN EVERYDAY LIFE
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Chemistry in every day life)|31 VideosD & F BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Aldehydes , Ketones, Carboxylic acid)|15 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-COORDINATION COMPOUNDS-Miscellneous Solved Problem (MSPS)
- Give the order of chelating effect of following ligands. (i) C(2)O(...
Text Solution
|
- Write the structural formula corresponding to each of the following IU...
Text Solution
|
- Write the IUPAC name of the following : (a) [Cr(acac)(3)] (b) [V(H(2...
Text Solution
|
- A solution containing 0.319 g of complex CrCl(3).6H(2)O was passed th...
Text Solution
|
- Calculate the effective atomic number of the metal atoms in the follow...
Text Solution
|
- Consider the following complexes: (I) K(2)Ptcl(6) (II) PtCl(4).2...
Text Solution
|
- Explain the following: (i) All the octahedral complexes of Ni^(2+)...
Text Solution
|
- You are given the following two complex X and Y which are isomer of ea...
Text Solution
|
- Allthe following complexes show a decrease in their weights when place...
Text Solution
|
- It is non experimental fact that Cs(2)[CuCl(4)] is orange coloured but...
Text Solution
|
- It is an experimental fact that : DMG+Ni(II)"salt"+NH(4)OH to Red prec...
Text Solution
|
- The correct order for the CFSE (numerical value ) for the following co...
Text Solution
|
- Which of the following statement is not correct ? (a) [Ni(H(2)O)(6)...
Text Solution
|
- Statement-1: [Co^(II)(NH)(3))(6)]^(2+) is not readily oxidized to [Co^...
Text Solution
|
- Which of the following is true about the complex [PtCl(2)(NH(3))(OH(2...
Text Solution
|
- Among [Ni(CO)(4)],[Ni(CN)(4)]^(2-),[NiCl(2)]^(2-) species, the hybridi...
Text Solution
|
- For the reaction Ni^(2+) +4NH(3) iff [Ni(NH(3))(4)]^(2+), at equilibri...
Text Solution
|
- In metal carbonyls the metal carbon bond length is found to be less th...
Text Solution
|
- pi-bonding is not involved in :
Text Solution
|
- Wilkinson's catalyst is
Text Solution
|