A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
RESONANCE ENGLISH|Exercise Exercise-2 Part-IV: Comprehension -1|5 VideosCOORDINATION COMPOUNDS
RESONANCE ENGLISH|Exercise Exercise-2 Part-IV: Comprehension -2|3 VideosCOORDINATION COMPOUNDS
RESONANCE ENGLISH|Exercise Exercise-2 Part-II: Single and double value integer Type|10 VideosCHEMISTRY IN EVERYDAY LIFE
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Chemistry in every day life)|31 VideosD & F BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Aldehydes , Ketones, Carboxylic acid)|15 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-COORDINATION COMPOUNDS-Exercise-2 Part-III: One of More than One Options Correct Type
- Which of the following statement(s) is/are incorrect?
Text Solution
|
- An effective atomic number of Co(CO)(4) is 35 annd hence is less stabl...
Text Solution
|
- Select the correct statement
Text Solution
|
- s-1: [MnCl(6)]^(3),[FeF(6)]^(3-) and [CoF(6)]^(-3) are paramagnetic ha...
Text Solution
|
- Which of the following is/are correctly matched ?
Text Solution
|
- Which of the following statement(s) is /are correct with respect to th...
Text Solution
|
- Spin only' magnetic moment of Ni in [Ni(dmg)2] is same as that found i...
Text Solution
|
- Which complex of the following pairs has the larger value of Delta(0) ...
Text Solution
|
- Which of the following isomerism is/are shown by the complex [CoCl(2)(...
Text Solution
|
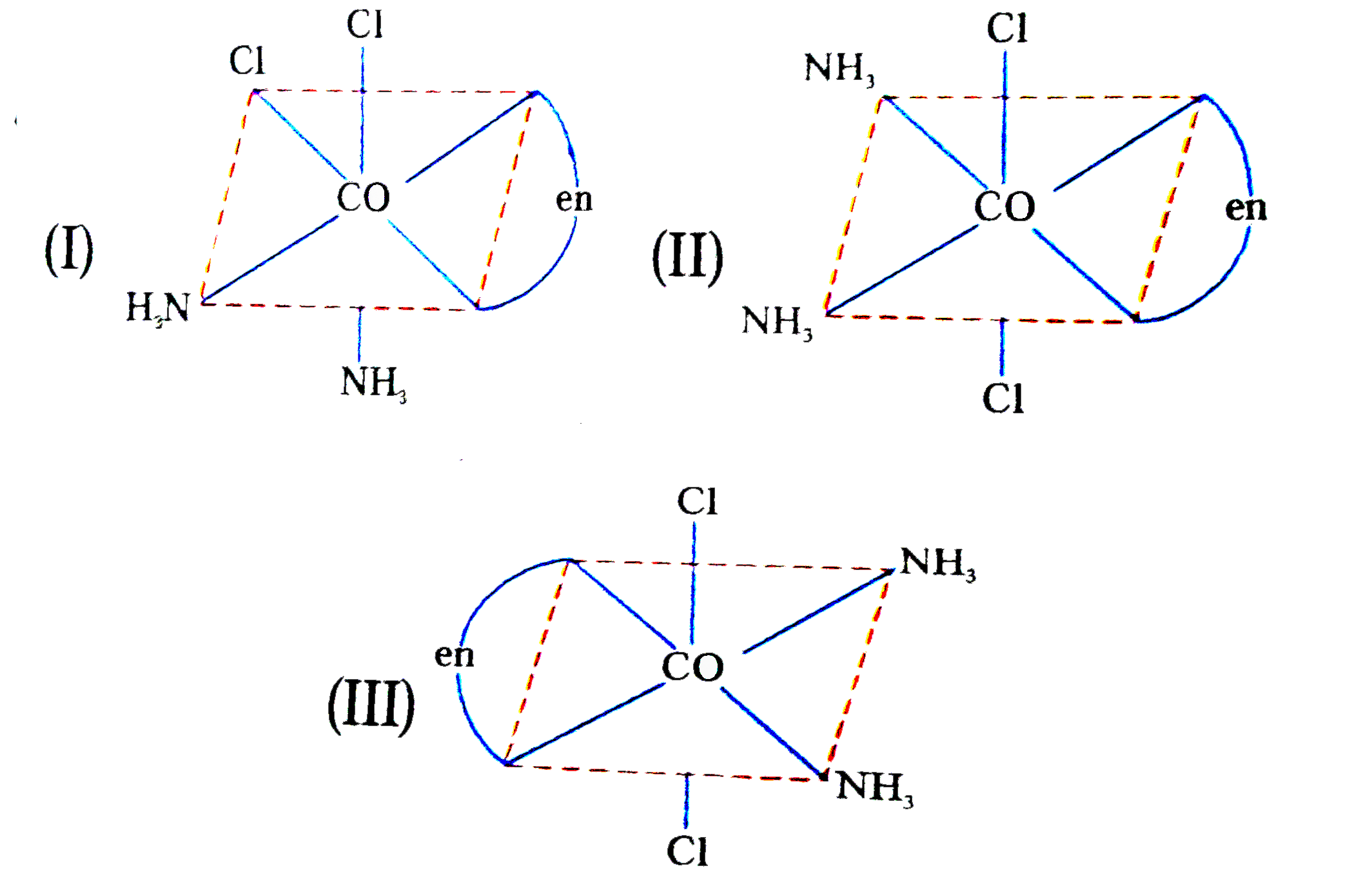
- Three arrangements are shown for the complex [Co(en)(NH(3))(2)Cl(2)]^(...
Text Solution
|
- Consider the following complexies [V(CO)(6)]^(-),[Cr(CO)(6)] and [Mn(C...
Text Solution
|
- Co forms dinuclear complex with a sigma bond with in two Cop atoms. C...
Text Solution
|