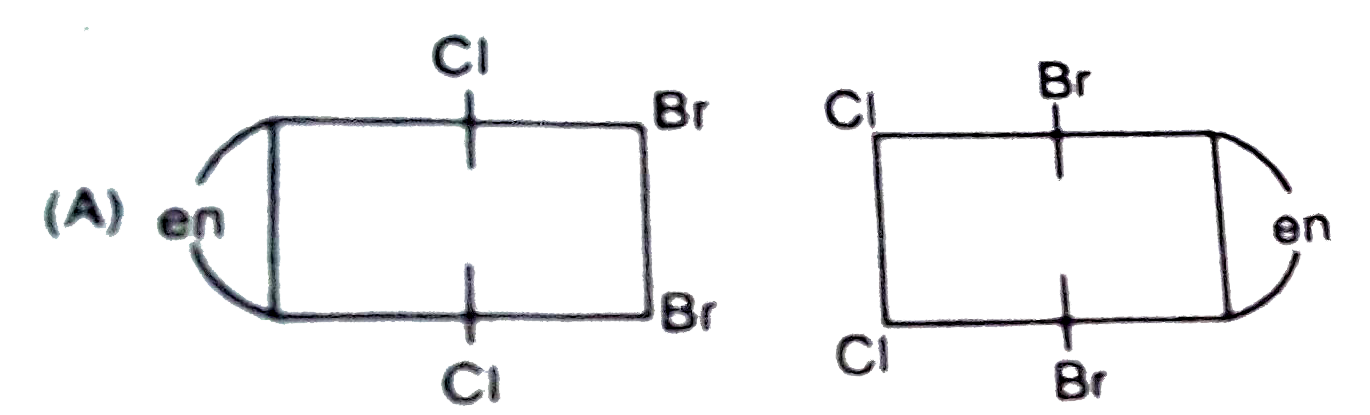
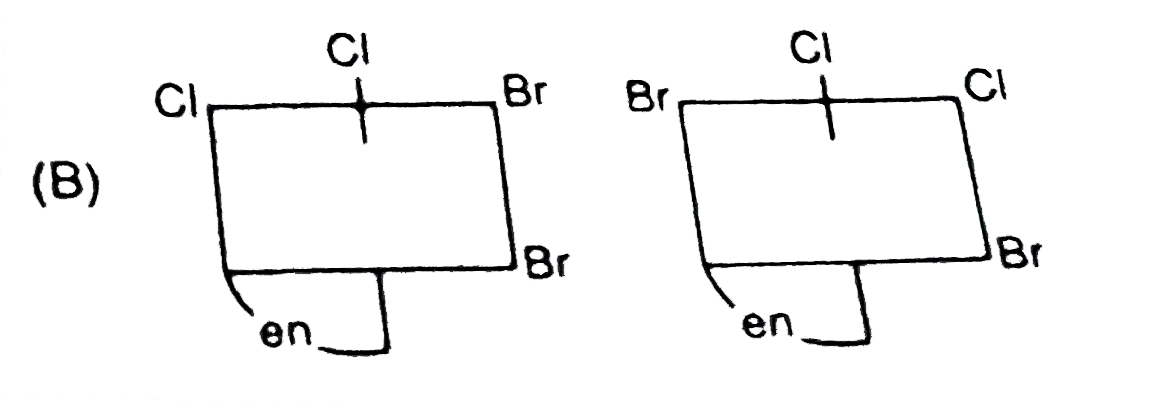
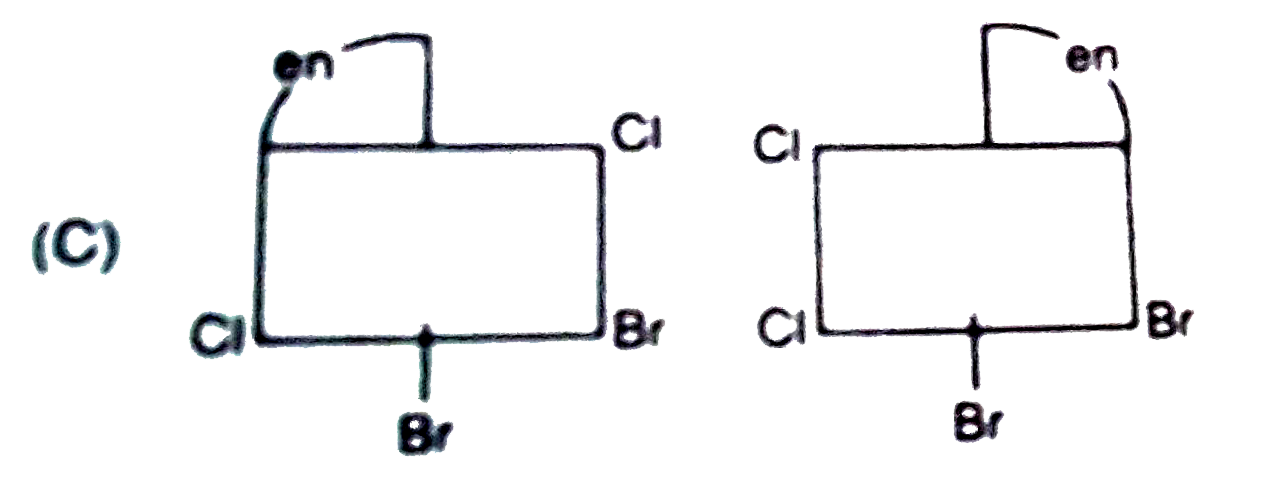
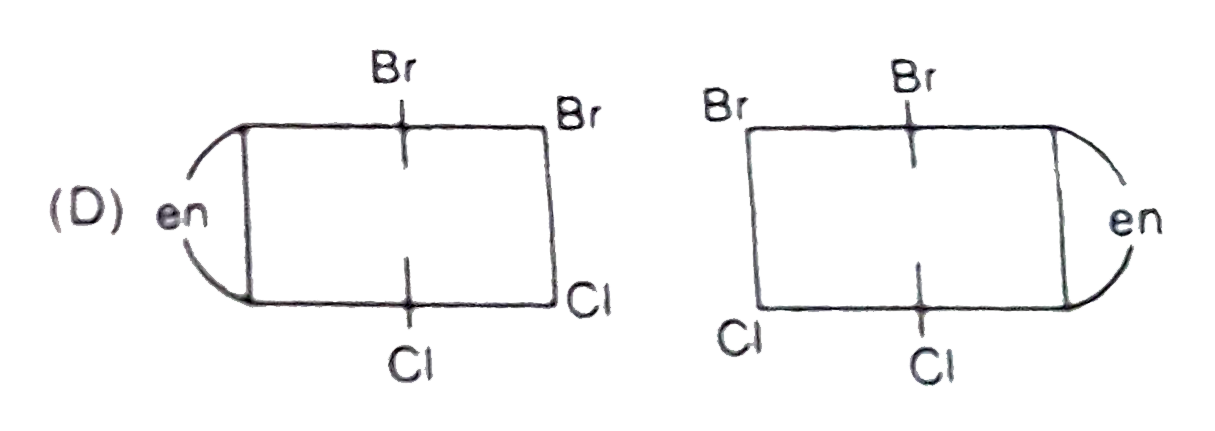
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
RESONANCE ENGLISH|Exercise Additional Problem for Self Practice (APSP) Part-III Subjected Question|7 VideosCOORDINATION COMPOUNDS
RESONANCE ENGLISH|Exercise Additional Problem for Self Practice (APSP) Part-III Only one option correct type|20 VideosCOORDINATION COMPOUNDS
RESONANCE ENGLISH|Exercise Additional Problem for Self Practice (APSP) Part-I|29 VideosCHEMISTRY IN EVERYDAY LIFE
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Chemistry in every day life)|31 VideosD & F BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Aldehydes , Ketones, Carboxylic acid)|15 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-COORDINATION COMPOUNDS-Additional Problem for Self Practice (APSP) Part-II
- Lattice energy of an ionic compound depends on
Text Solution
|
- IUPAC name of |Co(ONO)(NH(3))(5)Cl(2)| is
Text Solution
|
- Among the following complexes, the one which shows zero crystal field ...
Text Solution
|
- When any solution passess through a cation exchange resin that is in a...
Text Solution
|
- A person having osteoporosis is suffering from lead poisoning. Ethlene...
Text Solution
|
- The complex that can show optical activity is :
Text Solution
|
- For [FeF(6)]^(3-) and [CoF(6)]^(3-), the correct statement is
Text Solution
|
- Which of the following will produce red color when treated with (NH(4...
Text Solution
|
- Which of the following reactions is not correct ?
Text Solution
|
- In which of the following complexes the metal ion has the lowest ionic...
Text Solution
|
- Which of the following ion has magnetic moment is 3.87 BM :-
Text Solution
|
- IUPAC name of complex ion [CrCl2("ox")2]^(3-) is
Text Solution
|
- [Co(NH(3))(4)(NO(2))(2)]Cl exhibits
Text Solution
|
- Metal 'M' forms a carbonyl compound in which it is present in its lowe...
Text Solution
|
- The most suitable reagent for the conversion of Propanol to Propanal ...
Text Solution
|
- The complex which has no d-electron in the central metal atom is .
Text Solution
|
- The complex ion . [M(en)Br(2)I(2)]^(-1), has two optical isomers. Thei...
Text Solution
|
- The IUPAC name of the complex [P t (Br) (Cl) (NH(3)) (3) (NO(2))]Cl i...
Text Solution
|
- The C-O bond length is the shortest in:
Text Solution
|
- The spin-only magnetic moment of [Fe(NH(3))(6)]^(3+) and [FeF(6)]^(3-)...
Text Solution
|