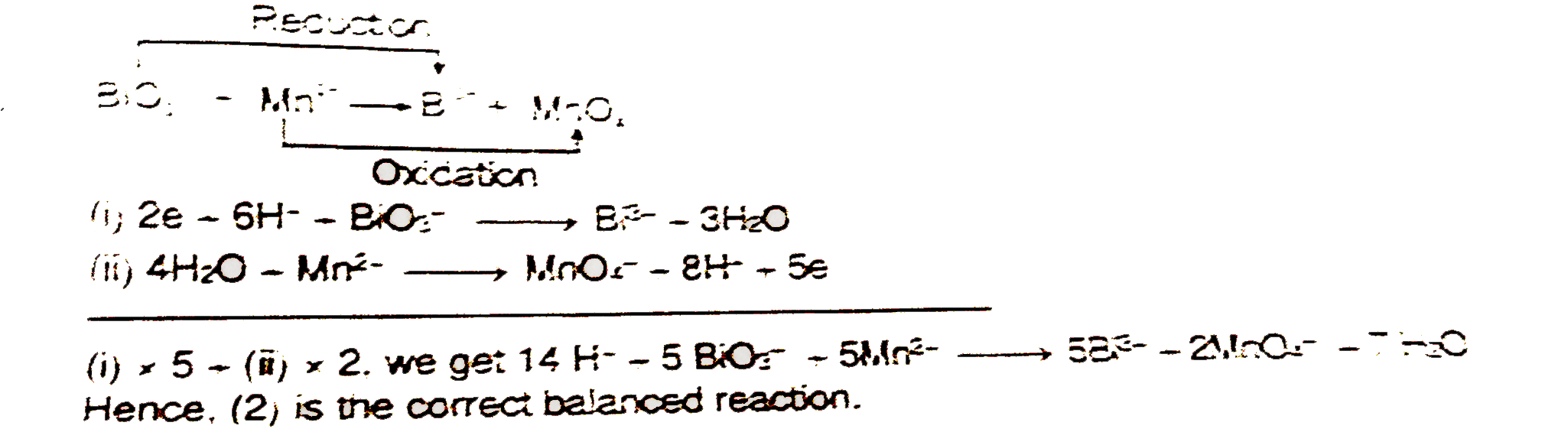A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
EQUIVALENT CONCEPT & TITRATIONS
RESONANCE ENGLISH|Exercise APSP (Part-II)|23 VideosEQUIVALENT CONCEPT & TITRATIONS
RESONANCE ENGLISH|Exercise APSP (Part-III)|12 VideosEQUIVALENT CONCEPT & TITRATIONS
RESONANCE ENGLISH|Exercise Exercise -3 (JEE(MAIN) Online problems)|10 VideosELECTROCHEMISRY
RESONANCE ENGLISH|Exercise Advanced Level Problems|88 VideosFUNDAMENTAL CONCEPT
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Fundamental Concept )|40 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-EQUIVALENT CONCEPT & TITRATIONS-APSP (Part-I)
- Calculate the mass of CaO required to remove the hardness of 1000,000 ...
Text Solution
|
- A 5.0 mL solution of H(2)O(2) liberates 1.27 g of iodine from an acidi...
Text Solution
|
- When hypo solution is added to KMnO(4) solution then
Text Solution
|
- Which of the following equations is a balanced one -
Text Solution
|
- 10ml of sulphuric acid solution (sp.gr.=1.84) contains 98% weight of p...
Text Solution
|
- The equivalent mass of MnSO4 is half of its molecular mass when it is ...
Text Solution
|
- An aqueous solution of 6.3 gm oxalic acid dihydrate is made upto Th...
Text Solution
|
- During the electrolysis of conc H(2)SO(4), it was found that H(2)S(2)...
Text Solution
|
- 1 mole of how many of the following acids neutralize exactly one mol ...
Text Solution
|
- CrO(5) has structure as shown The oxidation number of chromium in...
Text Solution
|
- The normality of orthophosphoric acid having purity of 70% by weight...
Text Solution
|
- Objective question (single correct answer). i. H(3) PO(4) is a triba...
Text Solution
|
- The reagent commonly used to determine hardness of water titrimetrical...
Text Solution
|
- 20 mL of 0.1 M solution of compound NaCO(3).NaHCO(3).2H(2)O is titrate...
Text Solution
|
- In the following reaction 2MnO(4^(-))+5H(2)O(2)^(18)+6H^(+)rarr2Mn^(2+...
Text Solution
|
- One gram equimolecular mixture of Na(2)CO(3) and NaHCO(3)is reacted wi...
Text Solution
|
- Which of the following is not a redox reaction ?
Text Solution
|
- Equivalent weight of chlorine molecule in the equation is : 3Cl(2)+6...
Text Solution
|
- Cr(2)O(7)^(2-)overset(H^(+))rarrCr^(3+), Eq. wt. of Cr(2)O(7)^(2-) is ...
Text Solution
|
- One mole of acidified K2Cr2O7 on reaction with excess KI will liberate...
Text Solution
|