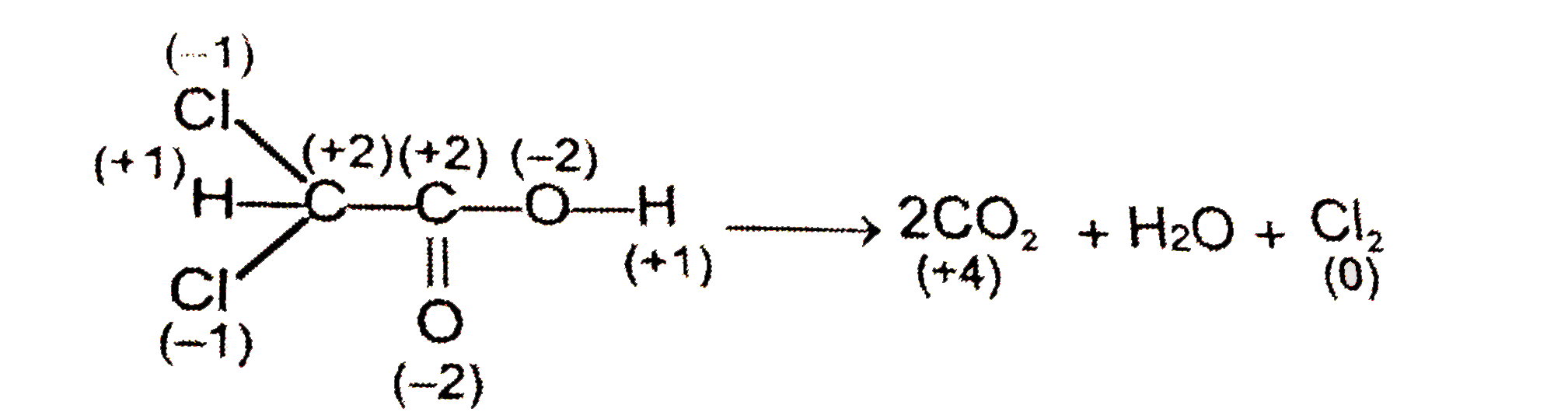
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
EQUIVALENT CONCEPT & TITRATIONS
RESONANCE ENGLISH|Exercise Part -IV|22 VideosEQUIVALENT CONCEPT & TITRATIONS
RESONANCE ENGLISH|Exercise APSP (Part-II)|23 VideosELECTROCHEMISRY
RESONANCE ENGLISH|Exercise Advanced Level Problems|88 VideosFUNDAMENTAL CONCEPT
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Fundamental Concept )|40 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-EQUIVALENT CONCEPT & TITRATIONS-APSP (Part-III)
- 0.1g of a solution containing Na(2)CO(3) and NaHCO(3) requires 10mL o...
Text Solution
|
- H(2) O(2) solution (20 mL) reacts quantitatively with a solution of KM...
Text Solution
|
- 0.70 g of mixture (NH4)2SO4 was boiled with 100 mL of 0.2 N NaOH solut...
Text Solution
|
- A mixture contaning 0.05 moleof K(2)Cr(2)O(7) and 0.02 "mole of" KMnO...
Text Solution
|
- If 100 mL of 1 N H(2)SO(4) is mixed with 100 mL of 1 M NaOH solution. ...
Text Solution
|
- 100 milli moles of dichloroacetic acid (CHCl(2)CO OH) can neutralize ...
Text Solution
|
- 1.2g of carbon is burnt completely in oxygen (limted supply) to produc...
Text Solution
|
- A mixutre solution of KOH and Na2CO3 requires 15 " mL of " (N)/(20) HC...
Text Solution
|
- Phenolphalein does not act as an indicator for the titration between
Text Solution
|
- A 1.00 g sample of H(2)O(2) solution containing X per cent H(2)O(2) by...
Text Solution
|
- A solution of H(2)O(2) labelled as '20 V' was left open. Due to this s...
Text Solution
|
- If amixture of Na(2)CO(3) and NaOH in equimolar quantities when react...
Text Solution
|