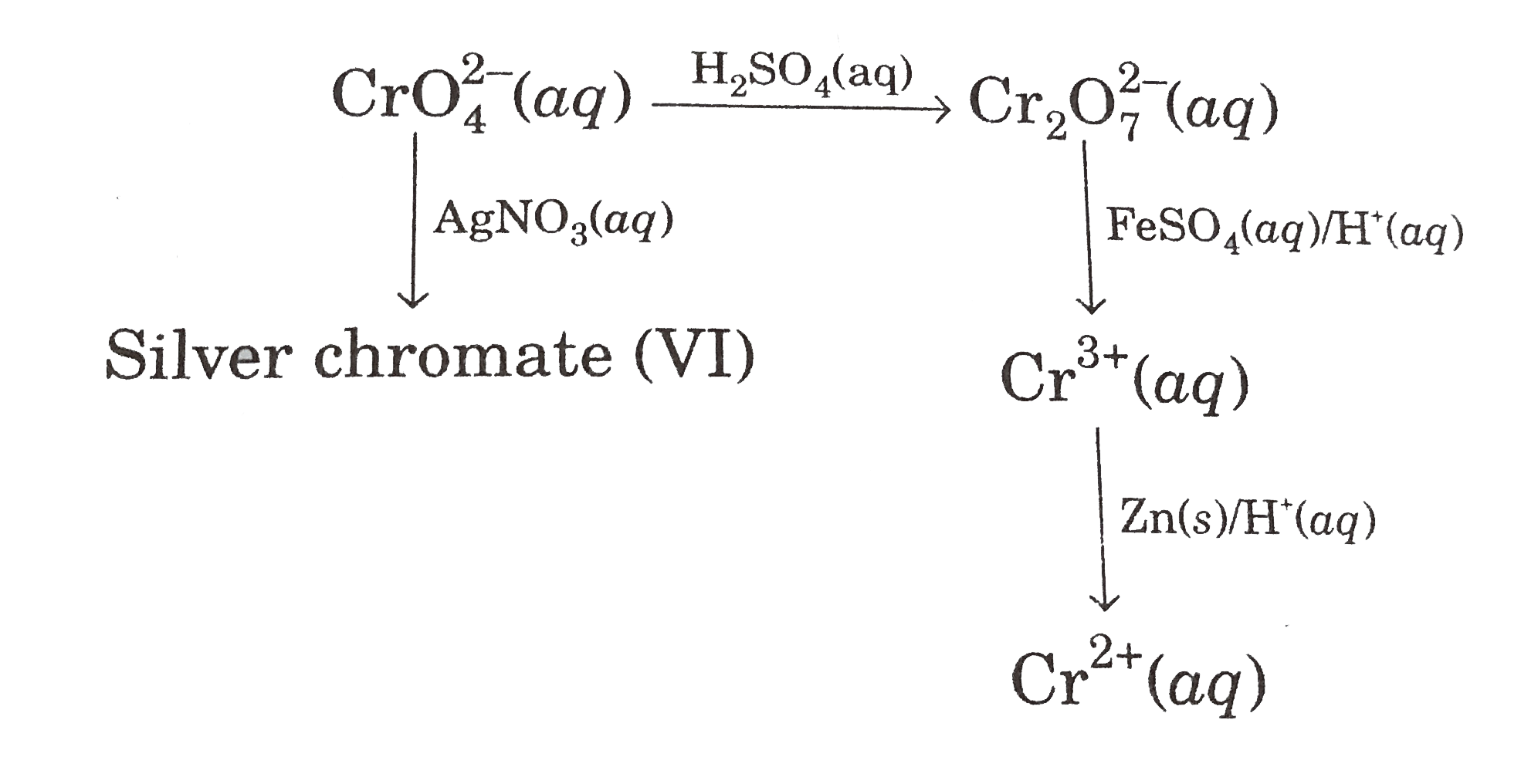A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-EQUIVALENT CONCEPT & TITRATIONS-Part -IV
- 10 mL of 1 NHCl is mixed with 20 mL of 1MH(2)SO(4) and 30 mL of 1M NaO...
Text Solution
|
- 20mL of H(2)O(2) after acidification with dilute H(2)SO(4) required 30...
Text Solution
|
- As(2)S(3) is
Text Solution
|
- During the titration of a mixture of Na(2)CO(3) and NaHCO(3) against H...
Text Solution
|
- In the reaction CrO(5) + H(2)SO(4)rarr Cr(2)(sO(4))(3)+H(2)O+O(2), one...
Text Solution
|
- Consider the redox reaction 2S(2)O(3)^(2-)+I(2)rarrS(4)O(6)^(2-)+2I^...
Text Solution
|
- Which of the following relations is/are correct for solutions ?
Text Solution
|
- Which of the following statements is/are correct
Text Solution
|
- H(2)C(2)O(4) and NaHC(2)O(4) behave as acids as well as reducing agent...
Text Solution
|
- Consider the reactions shown below : Which of the follwoing state...
Text Solution
|
- 3 " mol of "a mixture of FeSO(4) and Fe(2)(SO(4))(3) requried 100 " mL...
Text Solution
|
- A 7.1 g sample of bleaching powder suspended in H(2)O was treated with...
Text Solution
|
- If the number of N-atoms in 1 molecule of Hyponitrous acid is x and th...
Text Solution
|
- Find the valency factor (n) for NH(2)OH in given reaction : Fe^(3+)+...
Text Solution
|
- A solution of Na(2)S(2)O(3) is standardized iodometrically against 0.1...
Text Solution
|
- 2 moles of a mixture of O(2) and O(3) is reacted with excess of acidi...
Text Solution
|
- The overall equation for the reaction between sodium carbonate solutio...
Text Solution
|
- The overall equation for the reaction between sodium carbonate solutio...
Text Solution
|
- The overall equation for the reaction between sodium carbonate solutio...
Text Solution
|
- Give the monomer of the following 1) Teflon 2) Polystyrene 3) Ba...
Text Solution
|