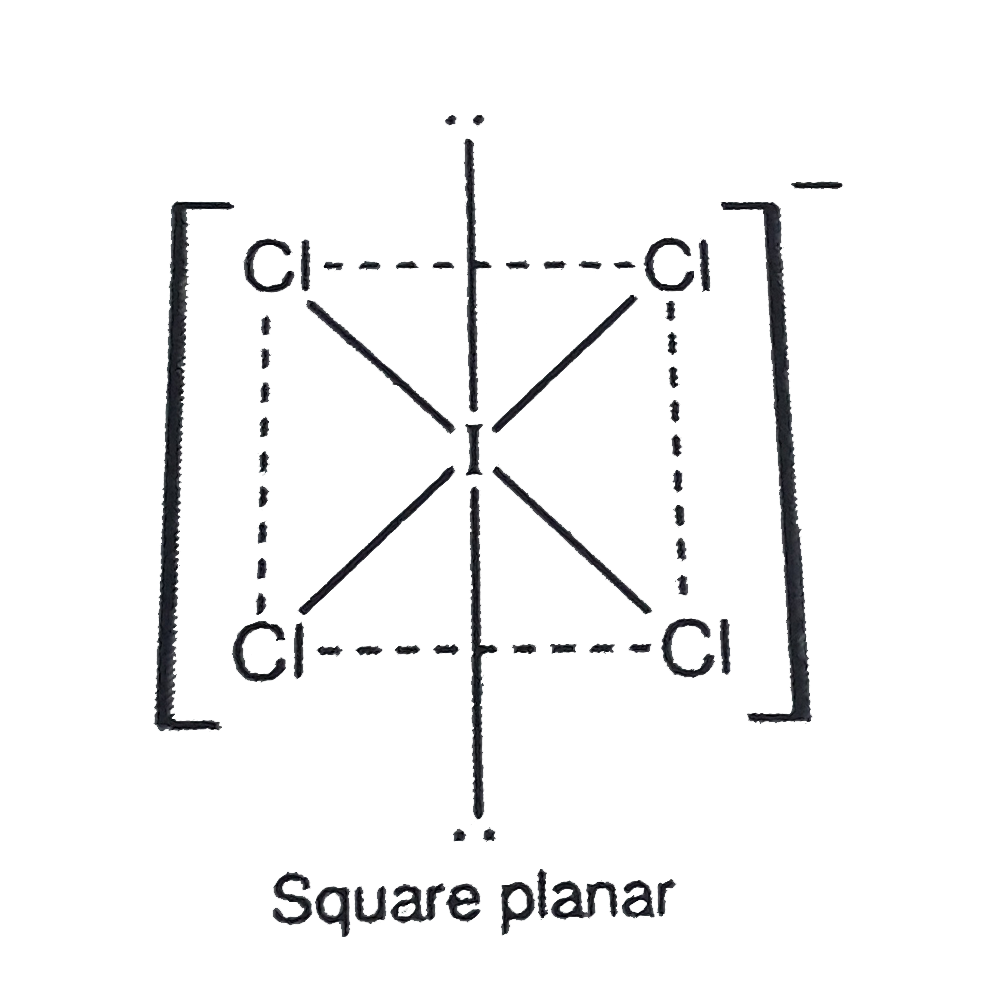
Text Solution
Verified by Experts
Topper's Solved these Questions
P-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)
RESONANCE ENGLISH|Exercise EXERCISE-1 PART-2|41 VideosP-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)
RESONANCE ENGLISH|Exercise EXERCISE-1 PART-3|3 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE ENGLISH|Exercise PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - V (INTERNATIONAL CHEMISTRY OLYMPIAD (IChO)) Problem 3|8 VideosPERIODIC TABLE & PERIODICITY
RESONANCE ENGLISH|Exercise Advanced Level Problems|105 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-P-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)-APSP PART-3
- Give the formula and describe the structure of a noble gas which is is...
Text Solution
|
- Which graph correctly describes a trend found in the halogen group ?
Text Solution
|
- ClO2 + Na2O2 rarr A+B (A) and (B) are:
Text Solution
|
- The following equilibria are given by rhoN(2)+3H(2)hArr2NH(3):K(1) ...
Text Solution
|
- Consider following properties of the noble gases. I. They readily fo...
Text Solution
|
- Consider equation: P4O10 + H2O → H3PO4. Number of moles of H3PO4 ...
Text Solution
|
- Cl(2)(g)+Ba(OH)(2)rarrX(aq.)+BaCl(2)+H(2)O X+H(2)SO(4)rarrY+BaSO(4) ...
Text Solution
|
- Which of the following on treatment with XeF6gives Xe ?
Text Solution
|
- Which of the following have melting point less than 298 K
Text Solution
|
- Identify A and B : PF(3)+H(2)O rarr A+HF, A overset∆ rarr B+ PH(3)
Text Solution
|
- Select the correct statement
Text Solution
|
- Which of the following are Pseudo halid
Text Solution
|
- Which of the following can be obtained by hydrolysis of XeF6
Text Solution
|
- What is the sum of group number and period number (according to IUPAC ...
Text Solution
|
- How many of the following compounds from HCl on hydrolysis as one of t...
Text Solution
|
- NH4ClO4 + HNO3 to HClO4 + (A), (A)oversetDeltato (B) (A) and (B)...
Text Solution
|
- The oxidation state of iodine in compound which is obtained by heating...
Text Solution
|
- NH4Cl + HNO3 to HCl + (A), (A)oversetDeltato (B) (A) and (B) are...
Text Solution
|
- The simplest ratio x:y of xenon and fluorine when passes through Ni-tu...
Text Solution
|
- White crystalline solid (A) reacts with H(2) to form a highly associat...
Text Solution
|
- White crystalline solid (A) reacts with H(2) to form a highly associat...
Text Solution
|