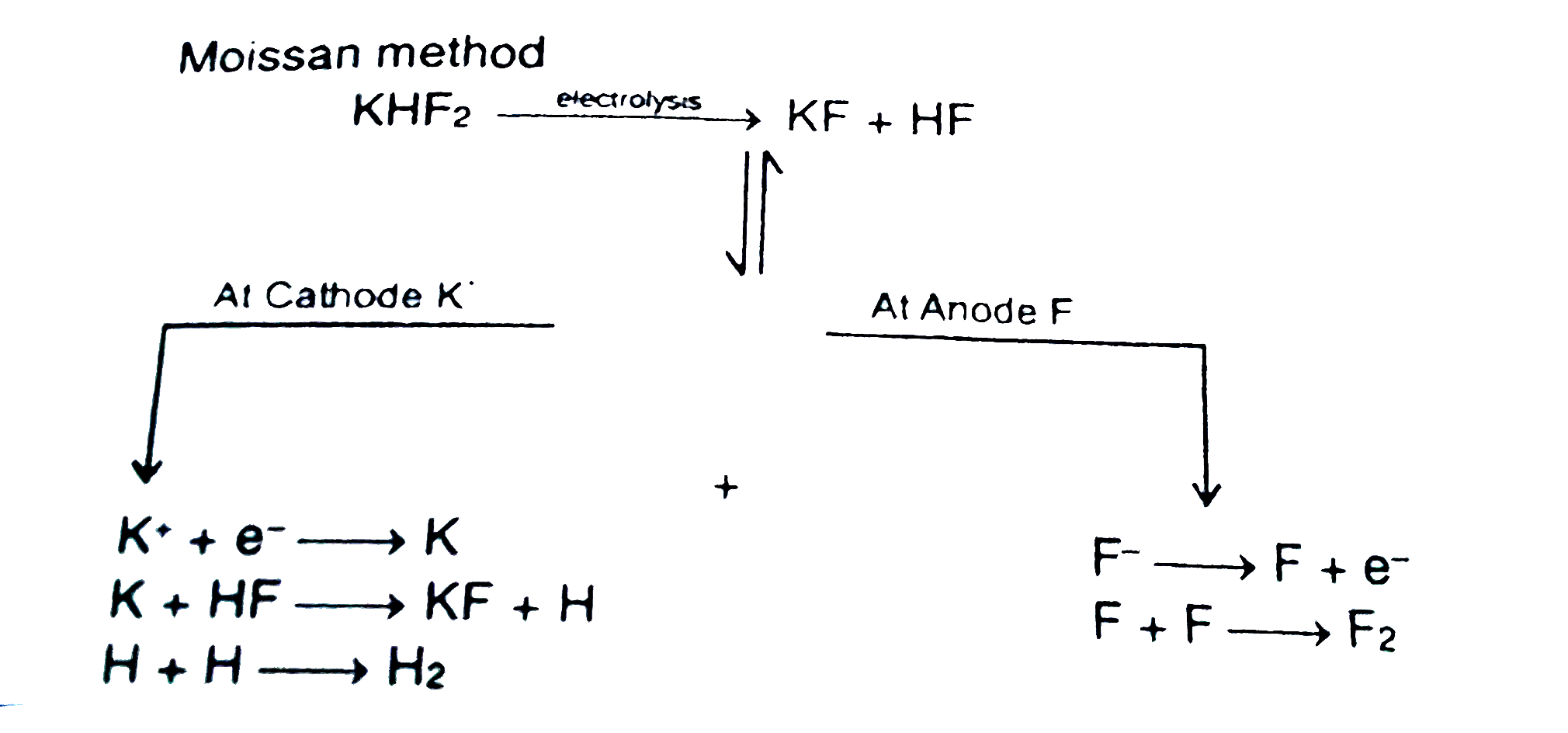A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)
RESONANCE ENGLISH|Exercise APSP PART-2|21 VideosP-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)
RESONANCE ENGLISH|Exercise APSP PART-3|22 VideosP-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)
RESONANCE ENGLISH|Exercise EXERCISE-3 PART-3|10 VideosP-BLOCK ELEMENT (BORON AND CARBON FAMILY)
RESONANCE ENGLISH|Exercise PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - V (INTERNATIONAL CHEMISTRY OLYMPIAD (IChO)) Problem 3|8 VideosPERIODIC TABLE & PERIODICITY
RESONANCE ENGLISH|Exercise Advanced Level Problems|105 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-P-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)-APSP PART-1
- The manufature of fluorine is done by :
Text Solution
|
- The catalyst used in the Deacon's process for the manufacture of chlor...
Text Solution
|
- Which electrolyte is used in Dennis method for the prepartion of fluor...
Text Solution
|
- Chlorine is liberted when we heat :
Text Solution
|
- An easy way of obtaining Cl2 gas in the laboratory is :
Text Solution
|
- When chlorine reacts with turpentine oil, the product formed is :
Text Solution
|
- Which of the following does not decolouries iodine ?
Text Solution
|
- In the reaction : 3Br(2)+6CO(3)^(2-)+3H(2)Orarr5Br^(-)+BrO(3)^(-)+6H...
Text Solution
|
- A greenish yellow gas reacts with an alkali metal hydroxide to form a ...
Text Solution
|
- Two gases X and Y bring about bleaching of flowers. X bleaches due to ...
Text Solution
|
- Which of the following gases can be dired by concentrated H2SO4
Text Solution
|
- Assertion: Conc. H(2)SO(4) cannot be used to prepare pure HBr from NaB...
Text Solution
|
- Which of the following is the weakest acid?
Text Solution
|
- Among the following which statement is not correct:
Text Solution
|
- Order of boiling point is:
Text Solution
|
- What is Euchlorine ?
Text Solution
|
- Consider the oxy acids HClO(pi) series, here value of n is 1 to 4. the...
Text Solution
|
- What happens when orthophosphoric acid is heated with nitric acid and...
Text Solution
|
- The strongest acid amongst the following is :
Text Solution
|
- Which of the following is not the characteristic of interhalogen compo...
Text Solution
|