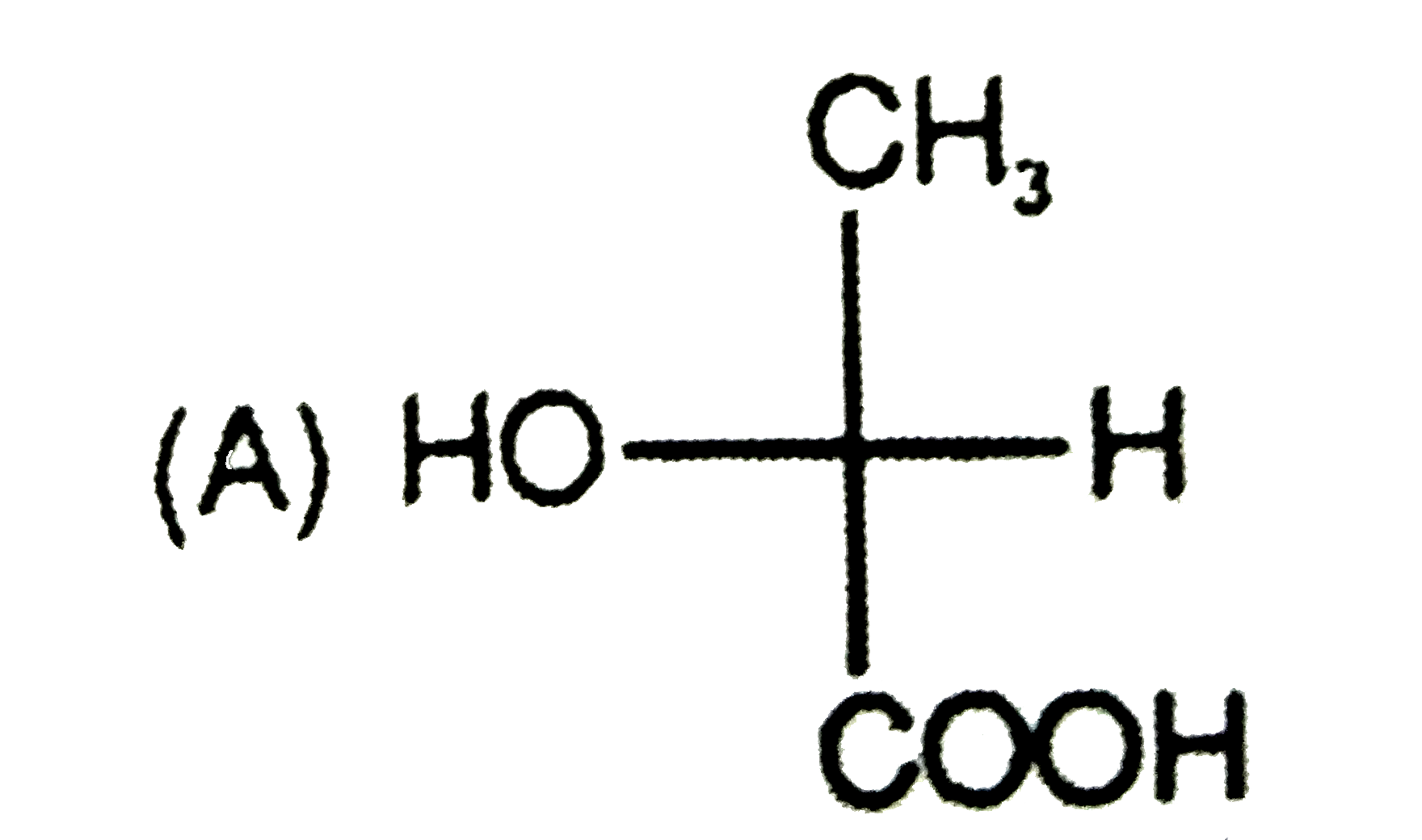
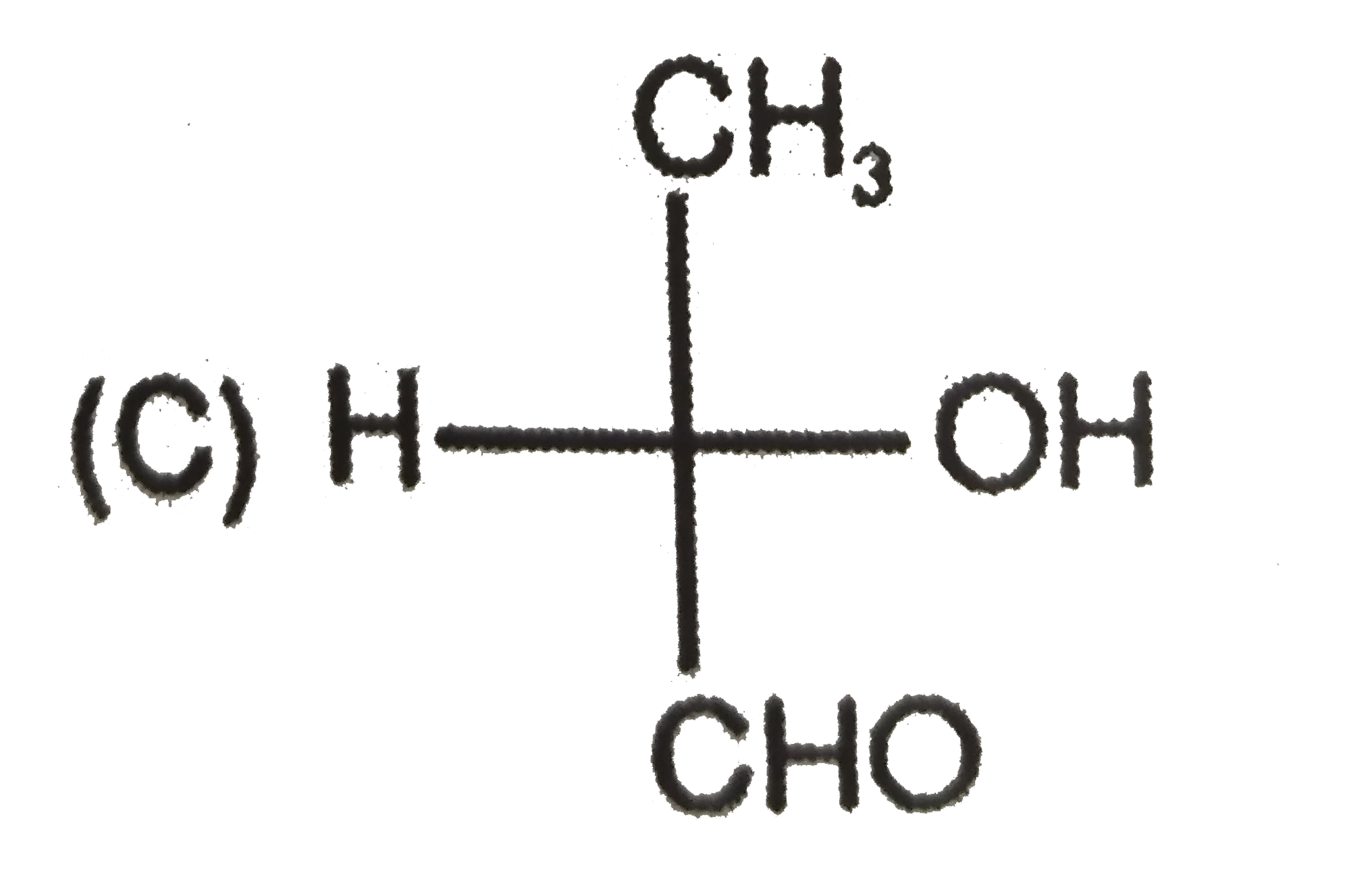
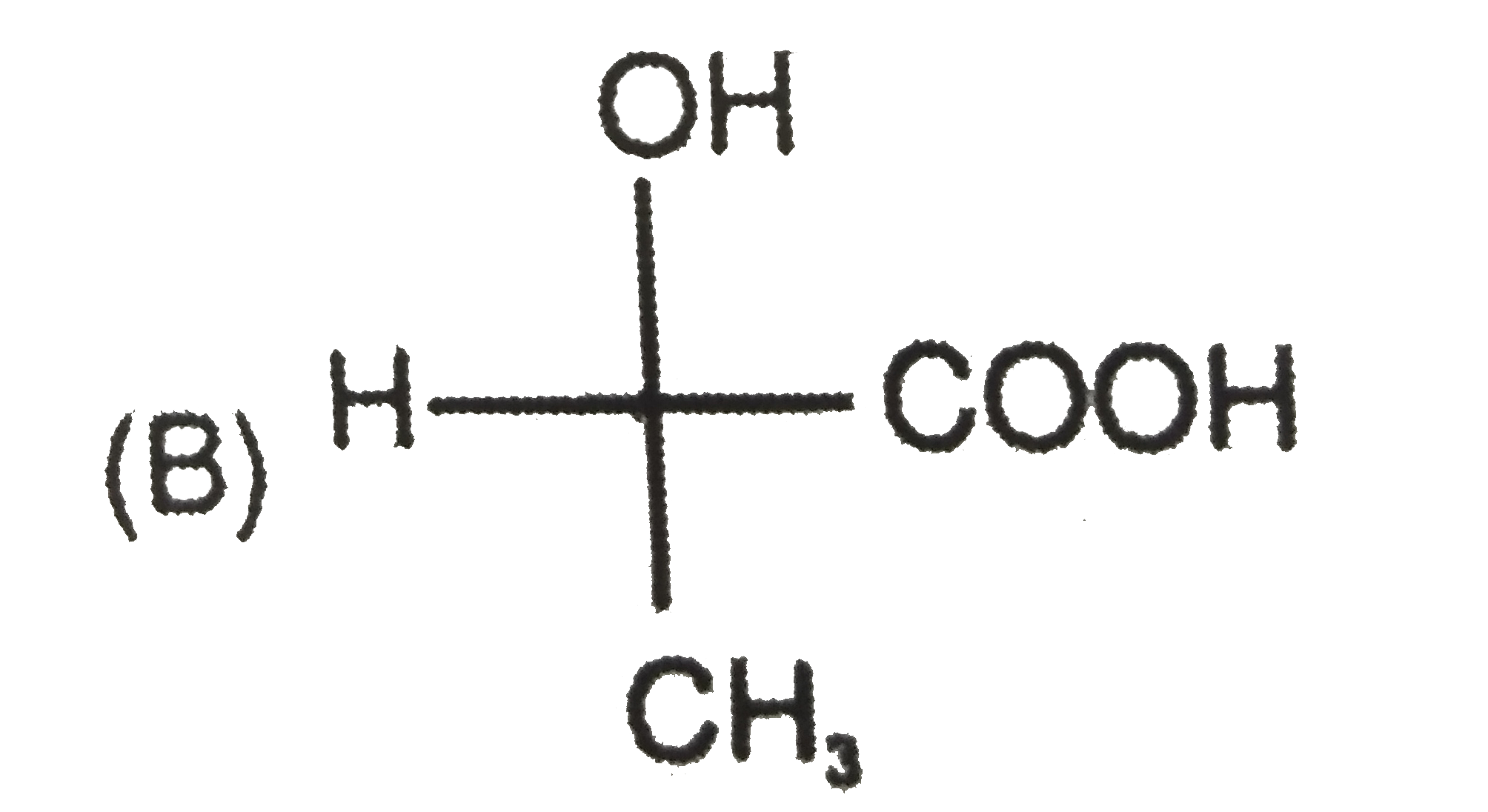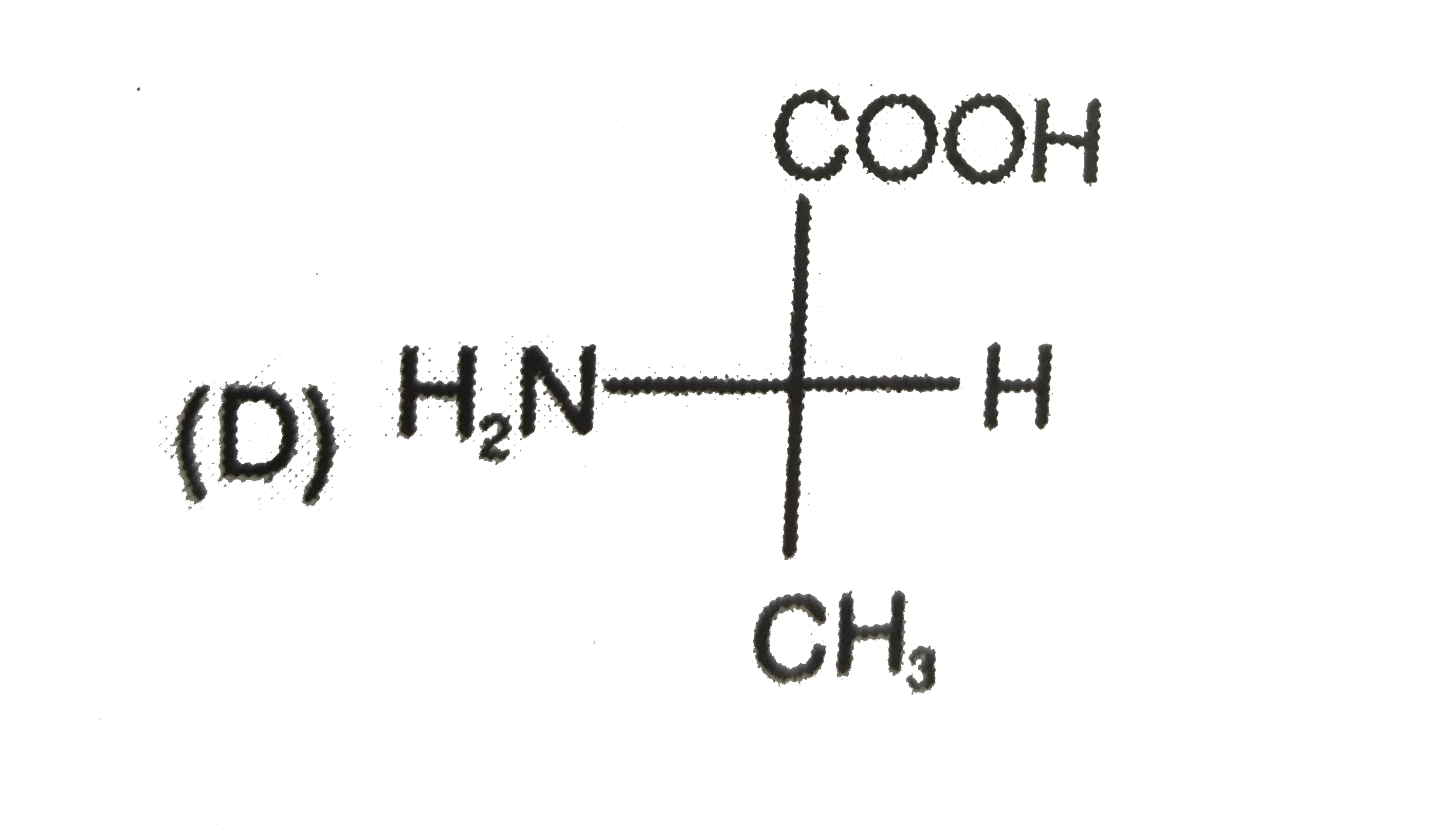To determine which compound has D configuration, we need to analyze the Fischer projections of the given compounds step by step. Here’s how we can do it:
### Step 1: Identify the Most Oxidized Carbon
In a Fischer projection, the most oxidized carbon (usually the carbonyl carbon in aldehydes or carboxylic acids) should be placed at the top.
**Hint:** Look for the functional groups; the carbon with the highest oxidation state should be at the top.
### Step 2: Draw the Fischer Projection
For each compound, draw the Fischer projection with the most oxidized carbon at the top. The remaining groups should be arranged around this carbon.
**Hint:** Ensure that the groups are arranged correctly based on their connectivity.
### Step 3: Determine the Position of the Hydroxyl Group (OH)
Once you have the Fischer projection, check the position of the hydroxyl group (OH) or amino group (NH2):
- If the OH or NH2 group is on the right side of the chiral carbon, it indicates D configuration.
- If the OH or NH2 group is on the left side, it indicates L configuration.
**Hint:** Focus on the chiral center and the orientation of the OH or NH2 group.
### Step 4: Analyze Each Compound
Now, let’s analyze each of the four compounds provided:
1. **First Compound:**
- Structure: OH, CH, COOH, CH3
- After rearranging, OH is on the right side → D configuration.
2. **Second Compound:**
- Structure: H, COOH, OH, CH3
- After rearranging, OH is on the left side → L configuration.
3. **Third Compound:**
- Structure: CH3, CHO, OH, H
- After rearranging, OH is on the left side → L configuration.
4. **Fourth Compound:**
- Structure: COOH, CH3, NH2, H
- After rearranging, NH2 is on the left side → L configuration.
### Conclusion
From the analysis, the first compound has D configuration, while the others have L configuration.
**Final Answer:** The first compound has D configuration.
---