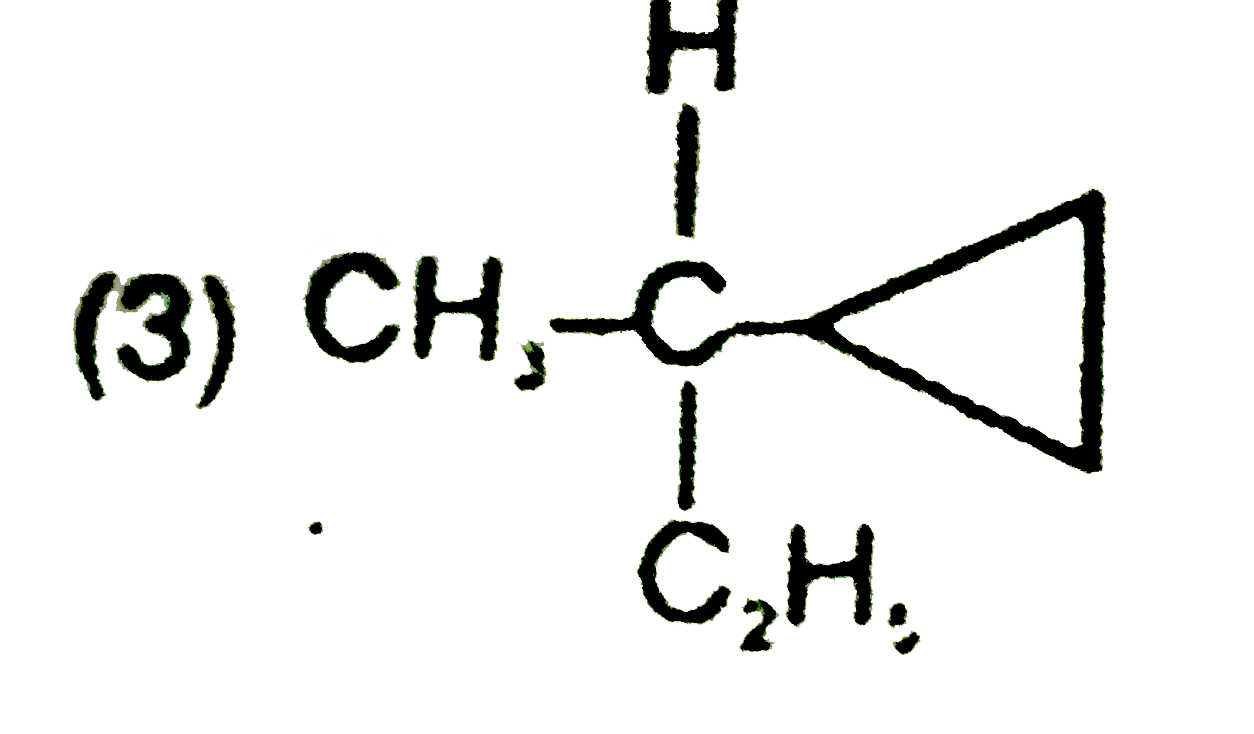
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
STEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (PART -II ONLINE JEE MAIN)|3 VideosSTEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (ADDITIONAL PROBLEMS FOR SELF PRACTICE (APSP)) PART 1|30 VideosSTEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (PART -1 JEE (ADVANCED))|16 VideosSOLUTIONS
RESONANCE ENGLISH|Exercise Advabced Level Problems (PART-2)|35 VideosSTRUCTURAL IDENTIFICATION
RESONANCE ENGLISH|Exercise Advanced level Problems (Part-III)|12 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-STEREOISOMERISM-EXERCISE (PART -II JEE (MAIN))
- Racemic mixture is formed ny mixing two:
Text Solution
|
- . Which of the following does not show geometrical isomerism?
Text Solution
|
- Among the following four structures I to IV I {:(" "CH(3))...
Text Solution
|
- Which of the following compounds will show meso isomer ?
Text Solution
|
- Amongst the following compounds, the optically active alkane having lo...
Text Solution
|
- Which of the following compounds is not chiral?
Text Solution
|
- Which type of isomerism is shown by 2,3-dichlorobutane ?
Text Solution
|
- Increasing order of stability among the three main conformation ( i.e....
Text Solution
|
- Which of the following molecules is expected to rotate the plane of pl...
Text Solution
|
- Which one of the following conformation of cyclohexane is chiral ?
Text Solution
|
- The absolute configuration of
Text Solution
|
- The alkene that exhibits geometrical isomerism is
Text Solution
|
- The number of stereoisomers possible for a compound of the molecular f...
Text Solution
|
- Out of the following, the alkene that exhibits optical isomerism is
Text Solution
|
- Which of the following compounds will exhibit geometrical isomerism?
Text Solution
|
- The absolute configuration of
Text Solution
|