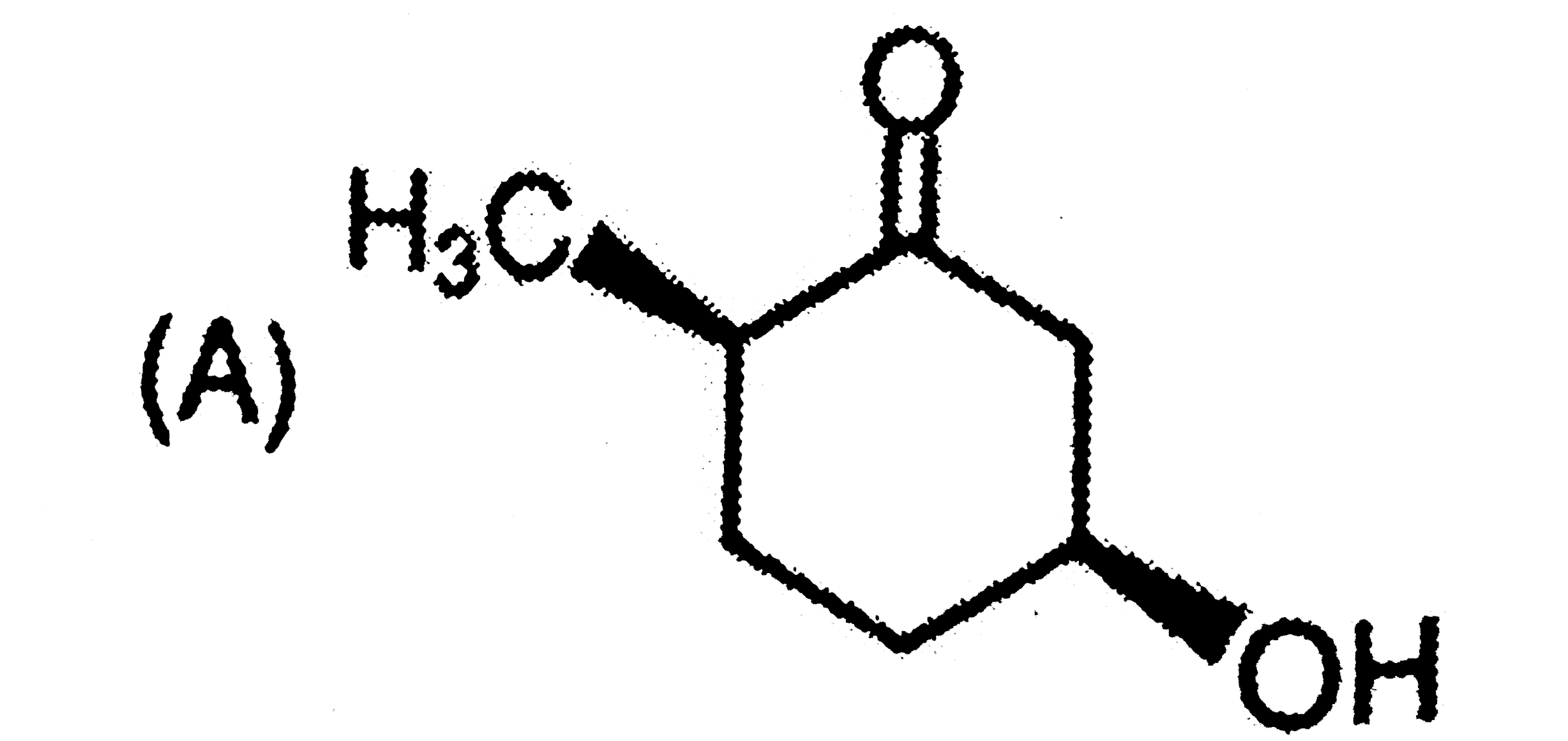
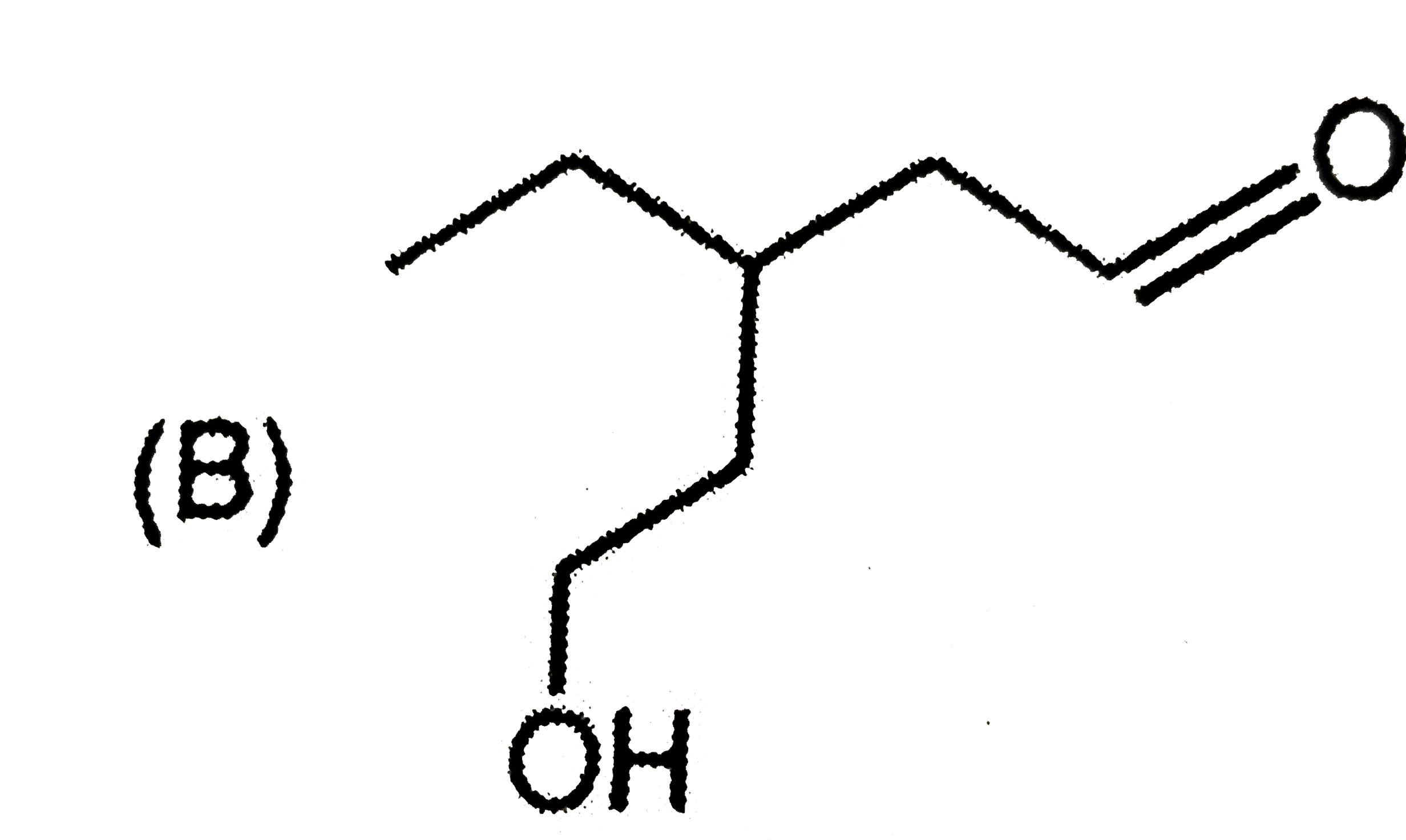
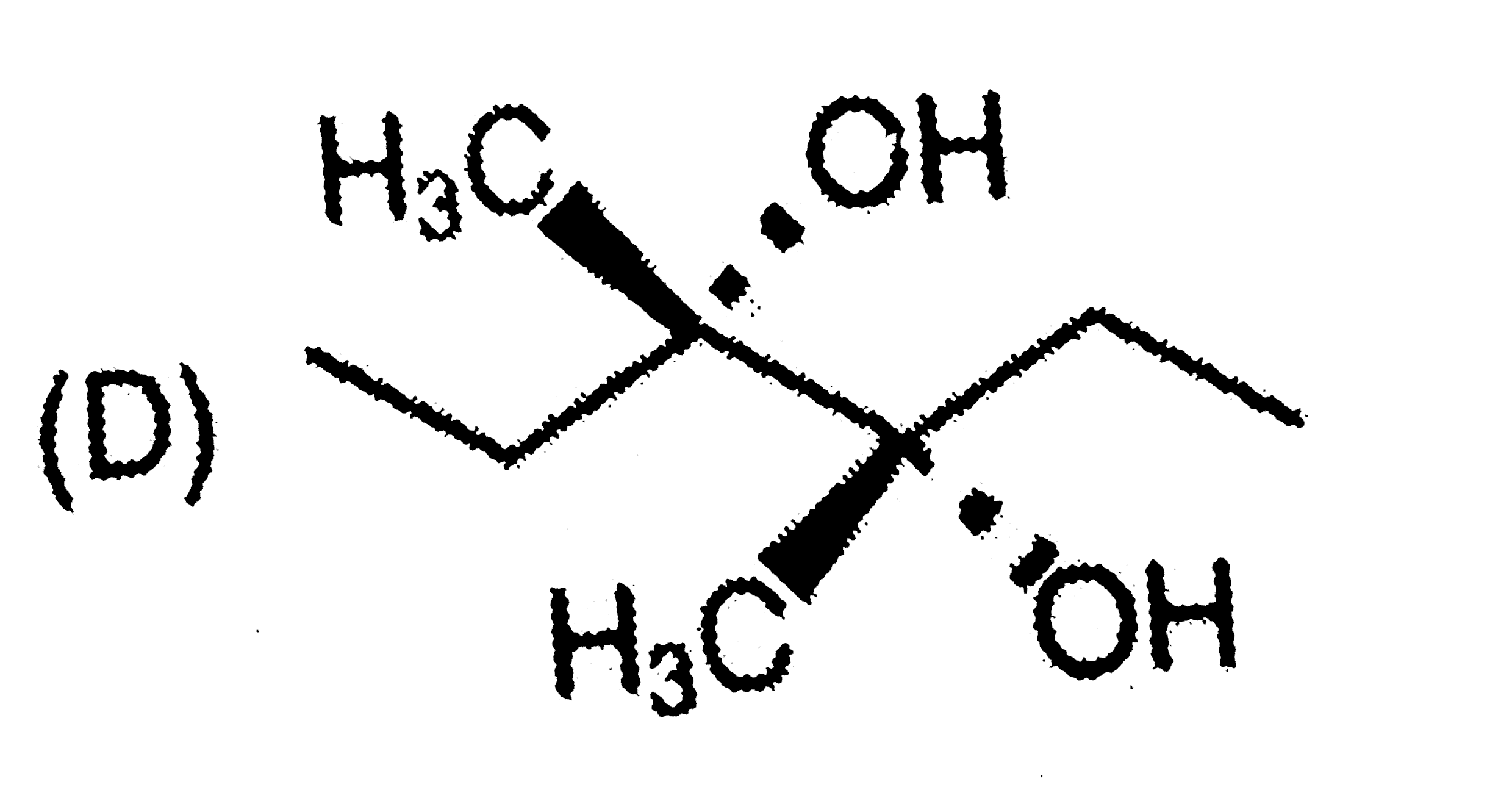
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
STEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (PART II : NATIONAL STANDARD EXAMINATION IN CHEMISTRY (NSEC) STAGE-1)|55 VideosSOLUTIONS
RESONANCE ENGLISH|Exercise Advabced Level Problems (PART-2)|35 VideosSTRUCTURAL IDENTIFICATION
RESONANCE ENGLISH|Exercise Advanced level Problems (Part-III)|12 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-STEREOISOMERISM-EXERCISE (PART III : PRACTICE TEST-2 (IIT-JEE (ADVANCED PATTERN))
- Which conformation of Bicyclo [2, 2, 2]-octane is more stable?
Text Solution
|
- In the given energy graph for cyclohexane, the point "B" represent.
Text Solution
|
- Identify the most stable stereoisomer :
Text Solution
|
- Molecular formula of smallest ester which contain one chiral carbon is...
Text Solution
|
- Correct the following names : 2-ol-2, 3-dimethylbutane
Text Solution
|
- Which statement(s) is/are correct for the given reaction and compounds...
Text Solution
|
- Intra-molecular H-bonding is possible in which of the following.
Text Solution
|
- Which of the following statement(s) is/are correct ?
Text Solution
|
- Which of the following compounds can show Optical isomerism as well as...
Text Solution
|
- Which of the following compound will show geometrical isomerism?
Text Solution
|
- Which of the following statement(s) is/are true about the following co...
Text Solution
|
- An organic compound P exists in two enantiomeric forms, which have spe...
Text Solution
|
- Total number of meso forms possible for 1,2,3,4 -Tetrachlorocyclobutan...
Text Solution
|
- If "A" is total number of meso compounds and "B" is total number of op...
Text Solution
|
- Sum of total no. of stereoisomers (A) and total no. of fractions (B) f...
Text Solution
|
- Which of the following carbonyl compound will give two products after ...
Text Solution
|
- Total number of stereoisomers of truxillic acid are :
Text Solution
|
- An unknown substance (P) shows optical activity. This optical activity...
Text Solution
|
- An unknown substance (P) shows optical activity. This optical activity...
Text Solution
|
- How will you Convert Phenol to Aniline?
Text Solution
|