Text Solution
Verified by Experts
Topper's Solved these Questions
NITROGEN & OXYGEN FAMILY
RESONANCE ENGLISH|Exercise Exercise 1 (Part-II : Only one option correct type)|50 VideosNITROGEN & OXYGEN FAMILY
RESONANCE ENGLISH|Exercise Exercise 1 (Part-III: Match the column)|2 VideosNITROGEN & OXYGEN FAMILY
RESONANCE ENGLISH|Exercise Part-III Practice Test-2 (JEE (Advanced Pattern))|23 VideosMETALLURGY
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Alkyl Halide, Alcohol,Phenol,Ether)|17 VideosNITROGEN CONTAINING COMPOUNDS
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Nitrogen containing Compounds)|30 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-NITROGEN & OXYGEN FAMILY-Exercise 1
- (a) Bond angle in PH(4)^(+) is higher than that in PH(3). Why? (b...
Text Solution
|
- Write the oxyacids of the following {:("Oxide","Oxyacids"),(N(2)O(3)...
Text Solution
|
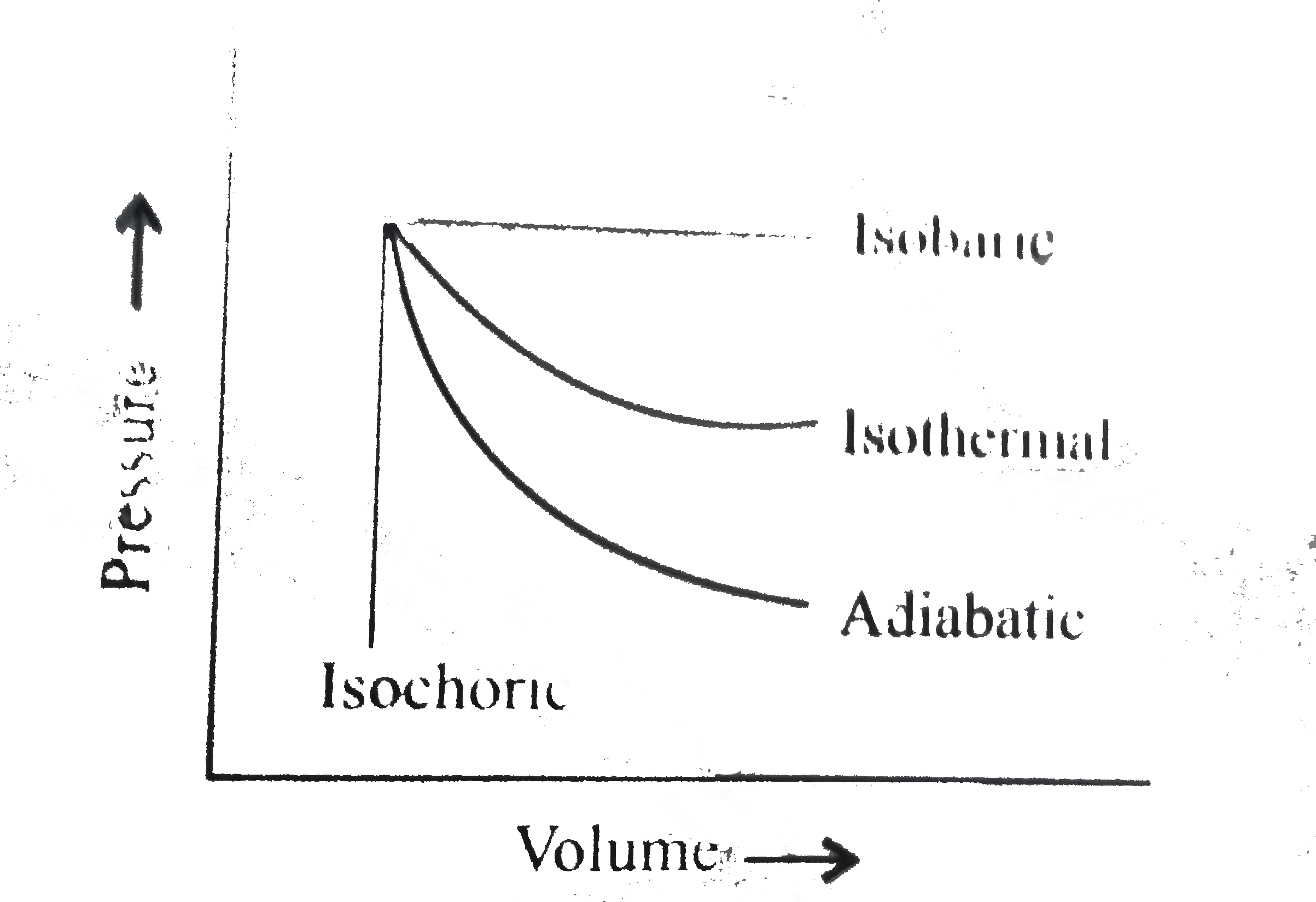
- The pressure-volume of various thermodynamic process is shown in graph...
Text Solution
|
- Write the oxyacids of the following oxides: (i) P(4)O(6) (ii) P(4)O(1...
Text Solution
|
- What happens when barium azide is heated ?
Text Solution
|
- Which stable elements of 15^(th) and 16^(th) group do not react with w...
Text Solution
|
- Chemiluminescence is a phenomenon in which on element glows in dark wh...
Text Solution
|
- Write the oxyacids of the following oxides: (i) SO(2) (ii) SO(3)
Text Solution
|
- When nitrogen dioxide gas is bubbled through water it produces
Text Solution
|
- Write balanced equation when NH(3) is dissolved in water.
Text Solution
|
- What happens when phosphine is absorbed in mercuric chloride solution ...
Text Solution
|
- On being slowly passed through water, PH3 forms bubbles but NH3 dissol...
Text Solution
|
- How is hydrazine prepared ?
Text Solution
|
- What is the product when PH(3) reacts with HNO(3) ?
Text Solution
|
- In the preparation of P(4)O(6), a mixture of N(2) and O(2) is used rat...
Text Solution
|
- A compound of 15^(th) group element is used as a fast drying agent in ...
Text Solution
|
- Write the oxyacids of the following oxides: N(2)O(3), N(2)O(5),N(2)O(4...
Text Solution
|
- Why does NO(2) dimerise?
Text Solution
|
- Why in the manufacture of H2 SO4 by contact process, sulphur trioxide ...
Text Solution
|
- How are SO(2) Cl(2).SO(3) and SO(2) obtained from sulphuric acid ?
Text Solution
|