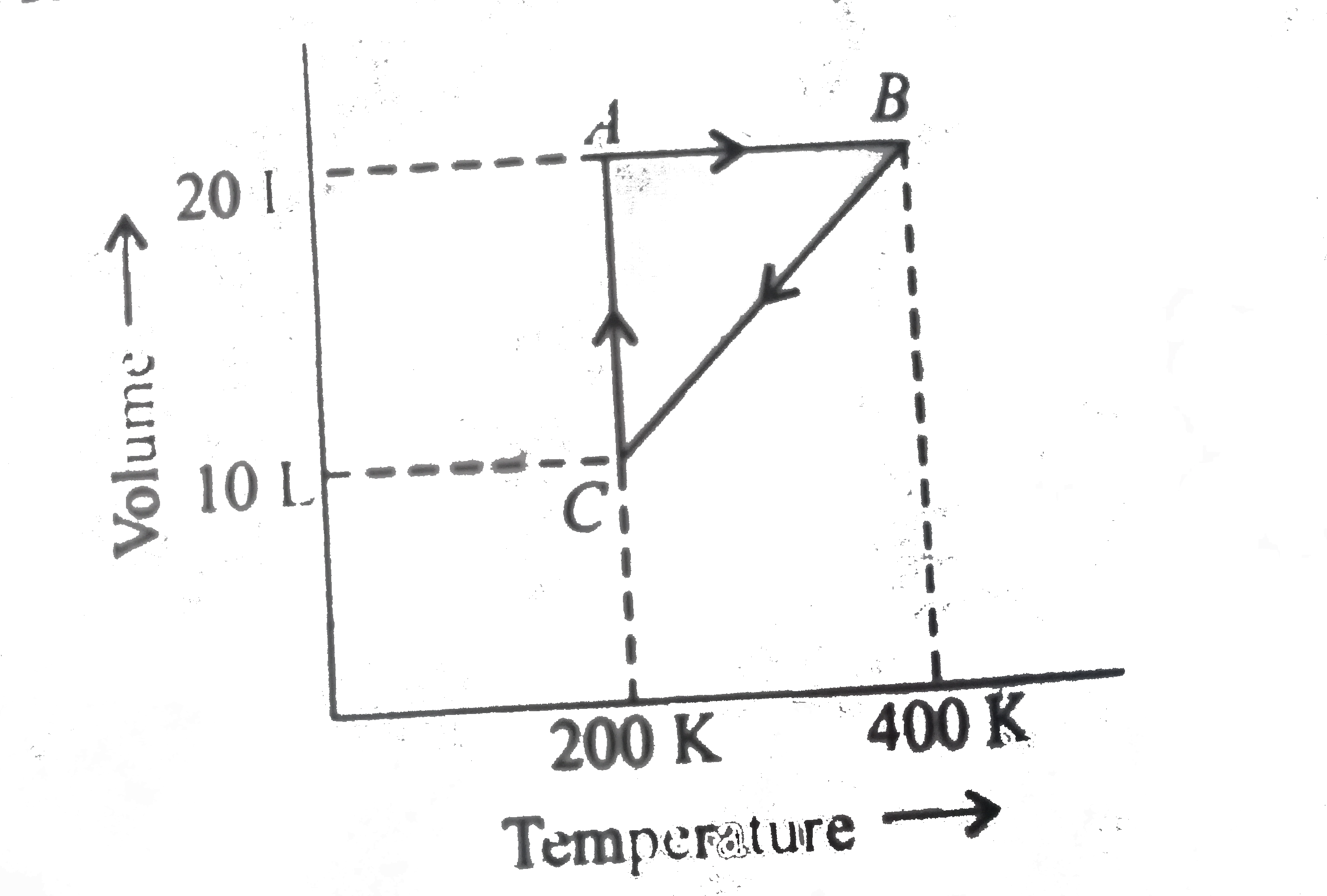
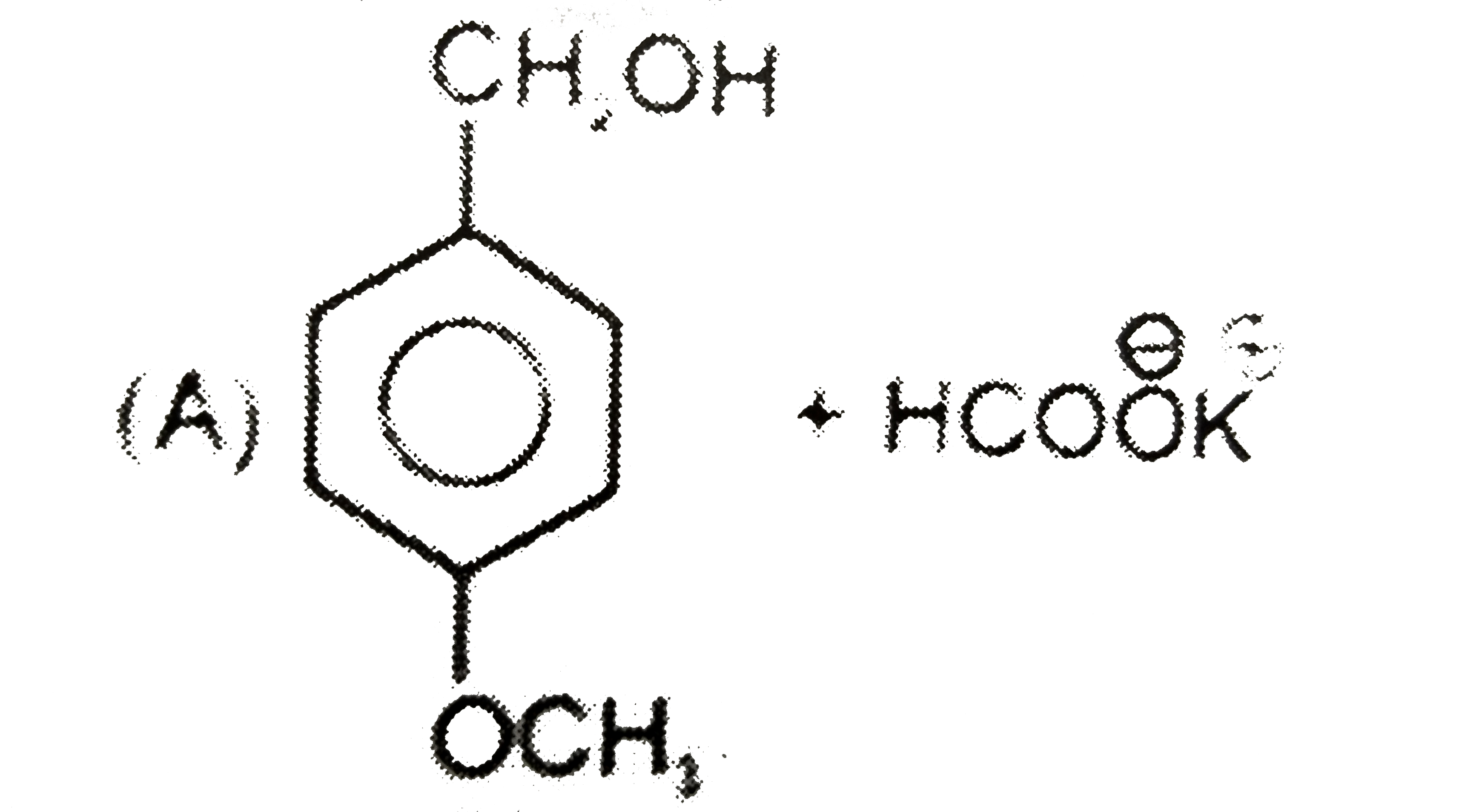
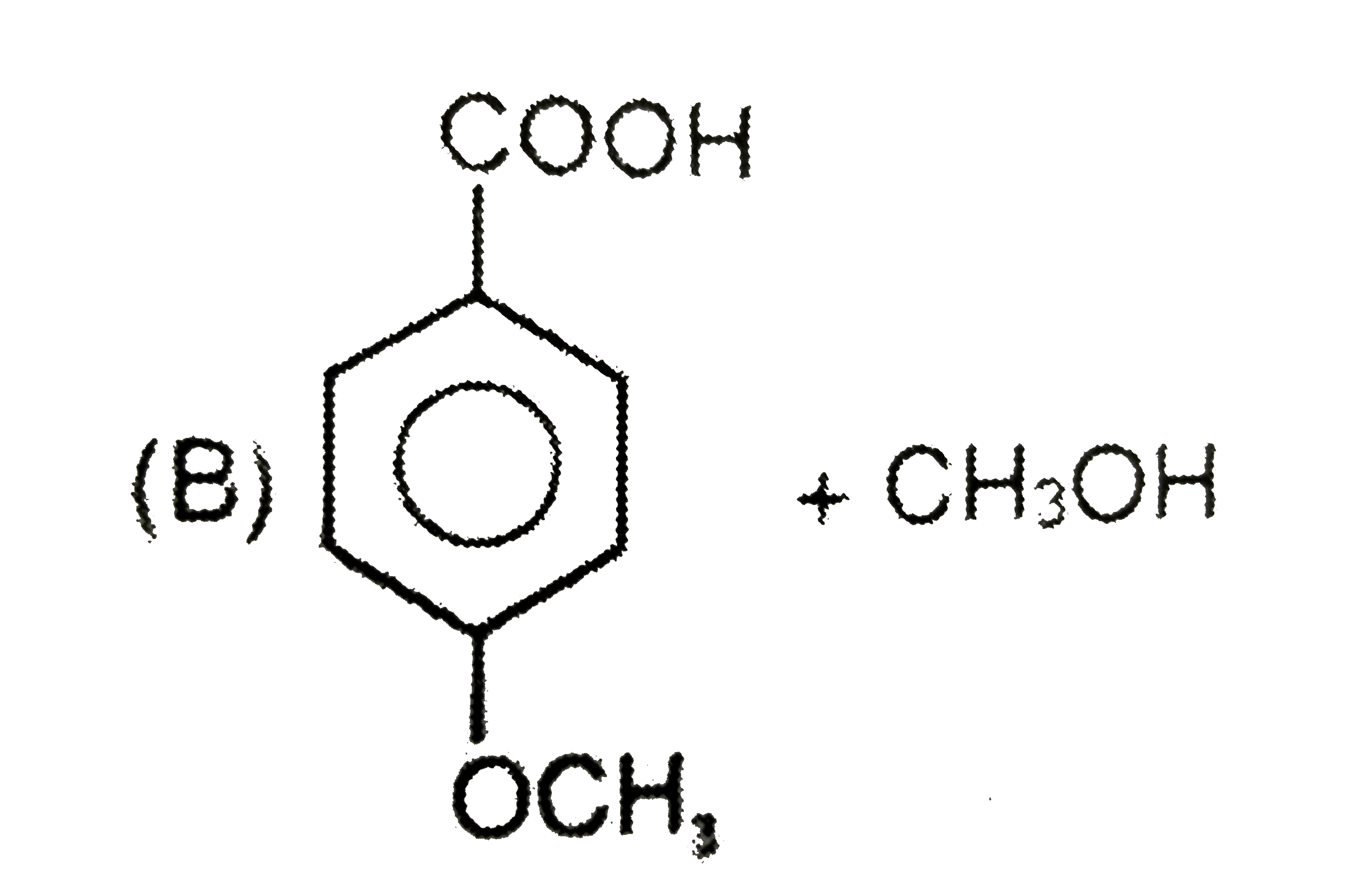
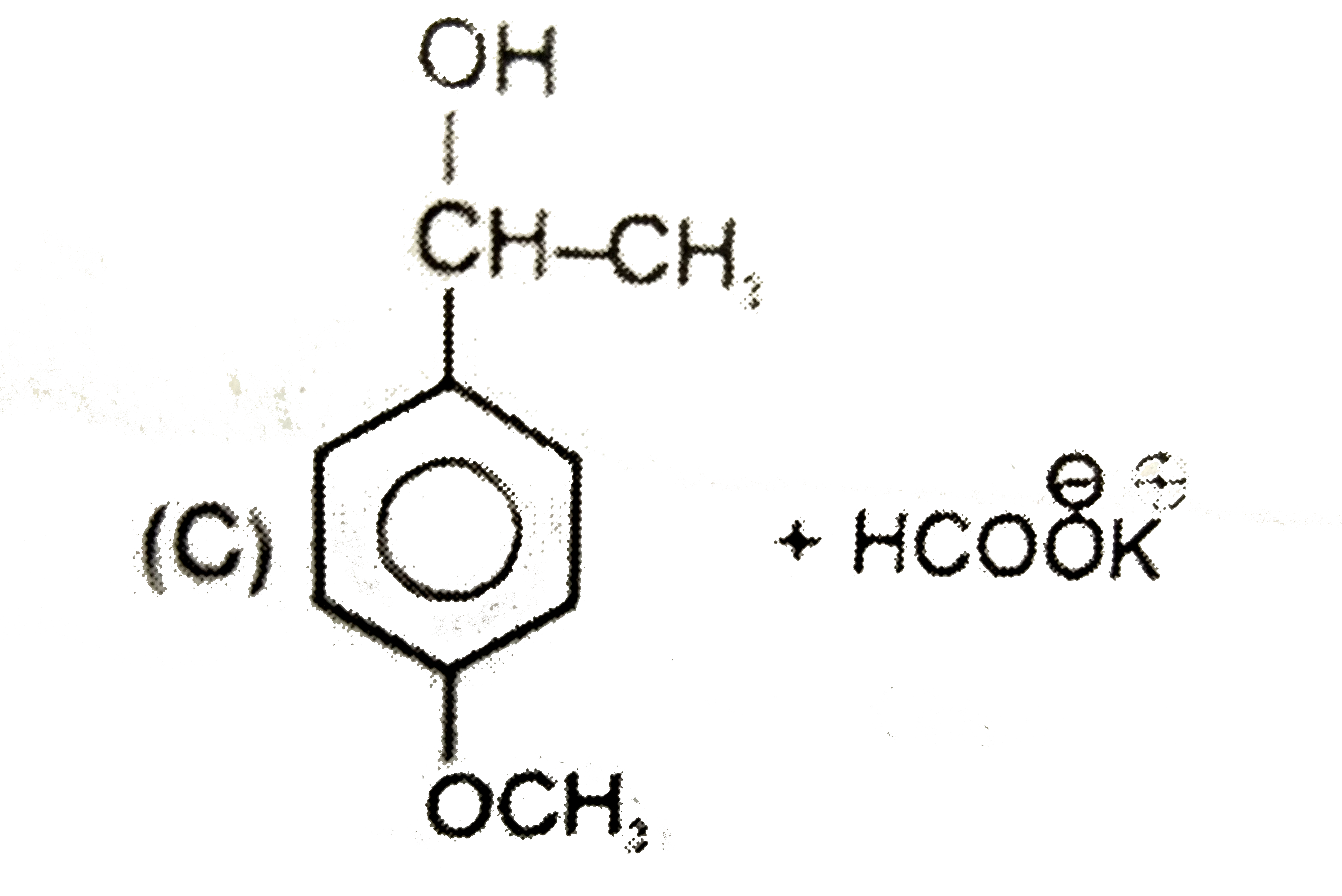
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise Part-III|8 VideosCARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise Exercise-2 Part-I|1 VideosCARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise Exercise-1 Part-II|1 VideosBIOMOLECULES & POLYMER
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Biomolecules & Polymer)|34 VideosCHEMICAL KINETICS
RESONANCE ENGLISH|Exercise PHYSICAL CHEMITRY (CHEMICAL KNIETICS & RADIOACTIVITY)|49 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID -Part-II
- What is the principal product of the following reactions?
Text Solution
|
- In the reaction , (CH(3))(3)C CHO +HCHO overset(NaOH)underset("heat")t...
Text Solution
|
- The pressures at A and B in the atmosphere are, respectively,
Text Solution
|
- In Cannizzaro's reaction, which intermediate ion is best hydride (H^(-...
Text Solution
|
- In the given reaction {:(" "CH(3)),(" |"),(H(3)C-C-...
Text Solution
|
- [X], product (X) in this reaction is
Text Solution
|
- Above conversion can be achieved by
Text Solution
|
- Major product is :
Text Solution
|
- In the following reaction final product is : C(6)H(5)MgBr +CO(2) ove...
Text Solution
|
- Which of the following does not give benoic acid salt on oxidation wit...
Text Solution
|
- The acid D obtained through the following sequence of reactions is C...
Text Solution
|
- In which of the following reaction the final product is neither an aci...
Text Solution
|
- Formic acid can be distinguish from acetic acid because formic acid
Text Solution
|
- Sodium bicarbonate reacts with salicylic acid to form
Text Solution
|
- Which of the following does not undergo Hell-Volhard-Zelinsky reaction...
Text Solution
|
- CH(3)CH(2)-CH(2)-COOH overset("Red P" +Br(2))toCH(3)-CH(2)-underset(B...
Text Solution
|
- What product is formed when acetic acid heated with P(2)O(5)
Text Solution
|
- Which of the following will not yeild a cyclic compound on heating
Text Solution
|
- RCOOAg+Br(2)overset(C C1(4))underset(Delta)rarrR-Br+AgBr+CO(2) this re...
Text Solution
|
- RCOOH to RCH(2)COOH. This conversion is known as reaction:
Text Solution
|