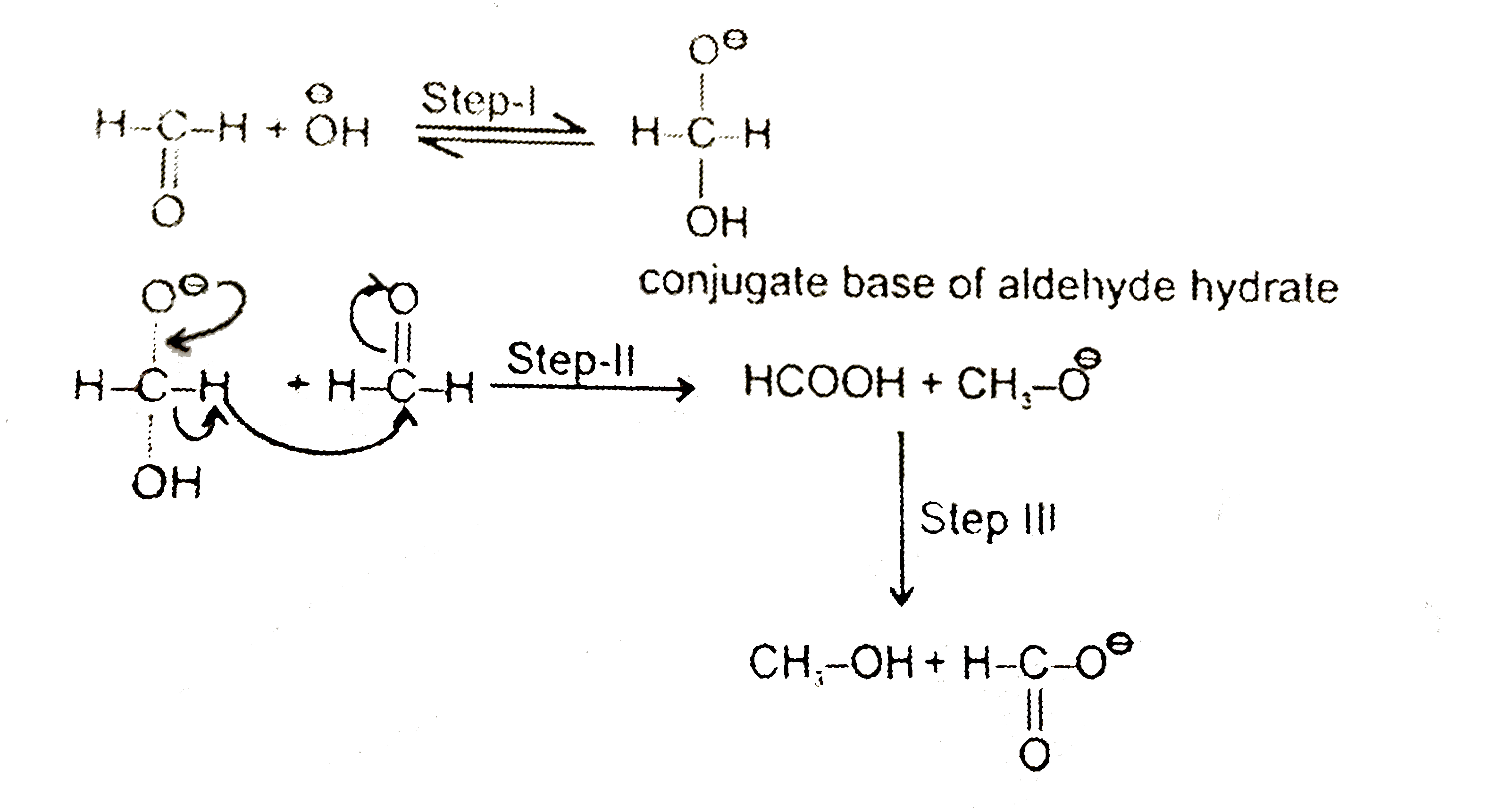A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise Exercise-3|1 VideosCARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise APSP|1 VideosCARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise Part-I|98 VideosBIOMOLECULES & POLYMER
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Biomolecules & Polymer)|34 VideosCHEMICAL KINETICS
RESONANCE ENGLISH|Exercise PHYSICAL CHEMITRY (CHEMICAL KNIETICS & RADIOACTIVITY)|49 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID -PART- IV
- Alldehydes and Ketones react with NH(2)OH to form aldoximes and Ketoxi...
Text Solution
|
- Aldehydes and Ketones react with NH(2)OH to form aldoximes and Ketoxim...
Text Solution
|
- Carbonyl compound which contains alpha-H gives aldol condenation react...
Text Solution
|
- Carbonyl compound which contains alpha-H gives aldol condenation react...
Text Solution
|
- Carbonyl compound which contains alpha-H gives aldol condenation react...
Text Solution
|
- The conversion of aldehyde having no alpha hydrogen to a mixture to a ...
Text Solution
|
- In the given reaction final product is
Text Solution
|
- Study following mecahnism of haloform reaction Which step is RDS
Text Solution
|
- Study following mecahnism of haloform reaction Whci of the follow...
Text Solution
|
- Study following mechanism of haloform reaction Which step produce...
Text Solution
|
- Which of the given combination is correct ?
Text Solution
|
- In which of the following combination , beta-hydroxy carbonyl is obtai...
Text Solution
|
- Define Binary and ternary Solution ?
Text Solution
|