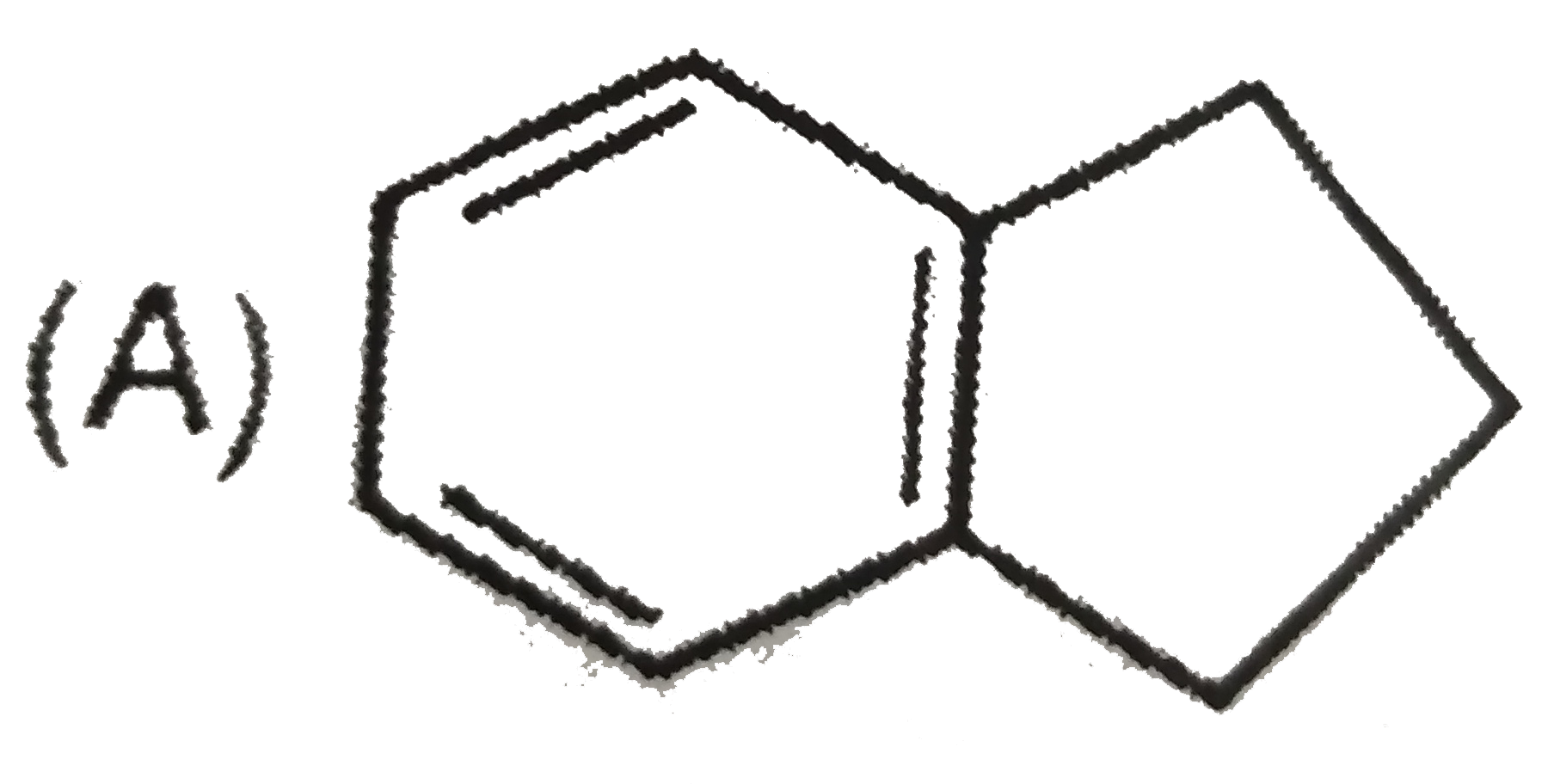
A
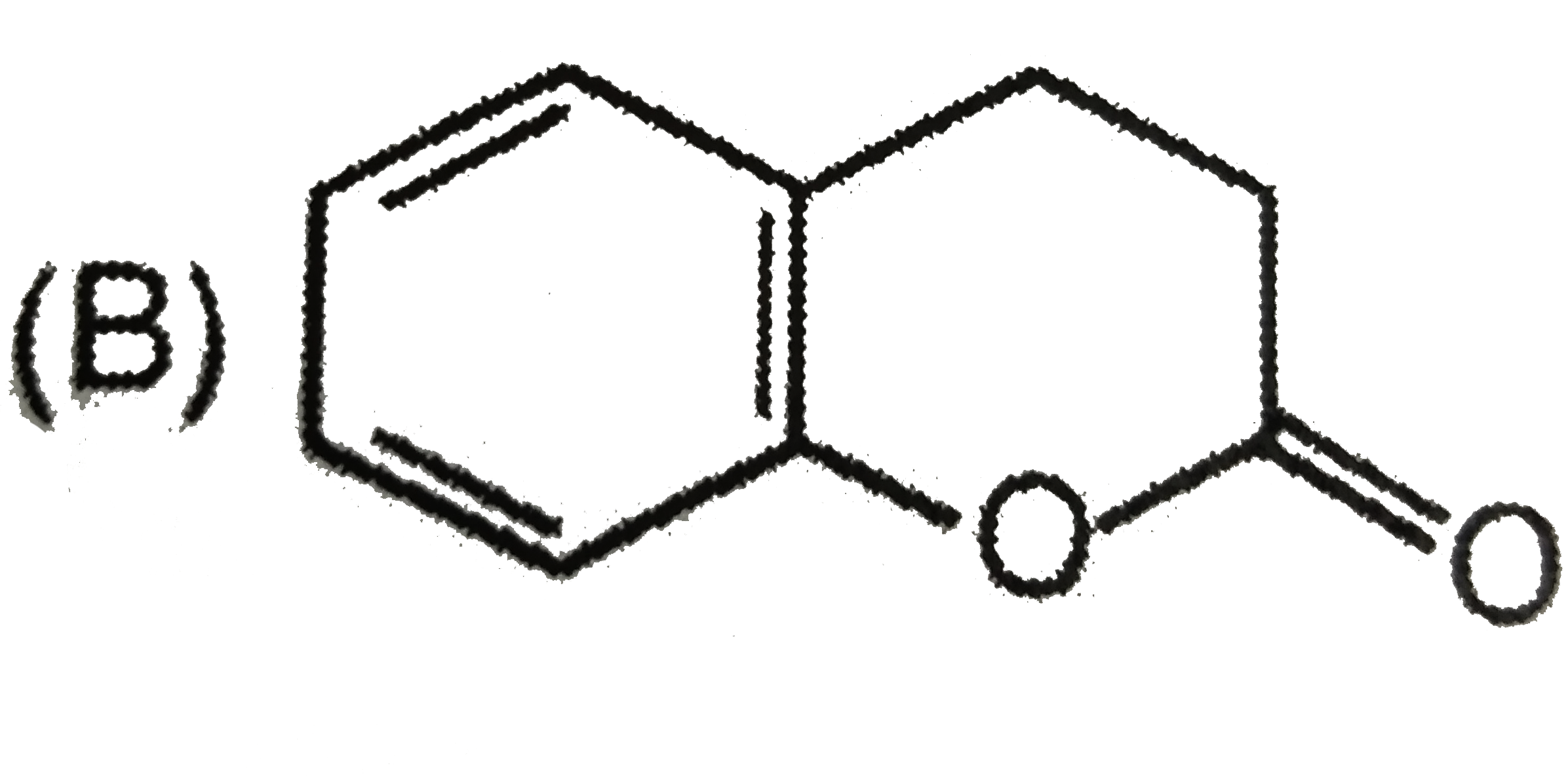
B
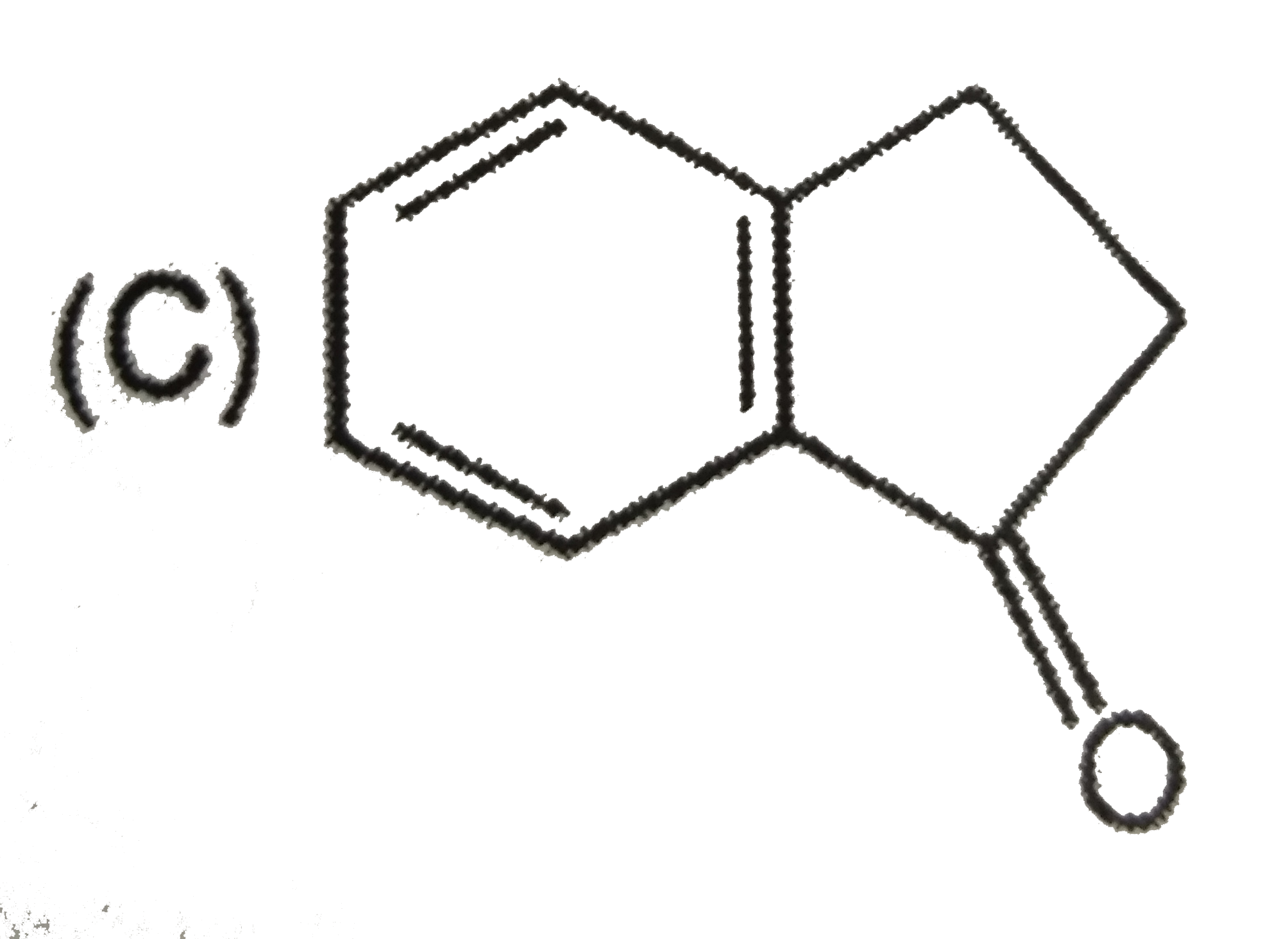
C
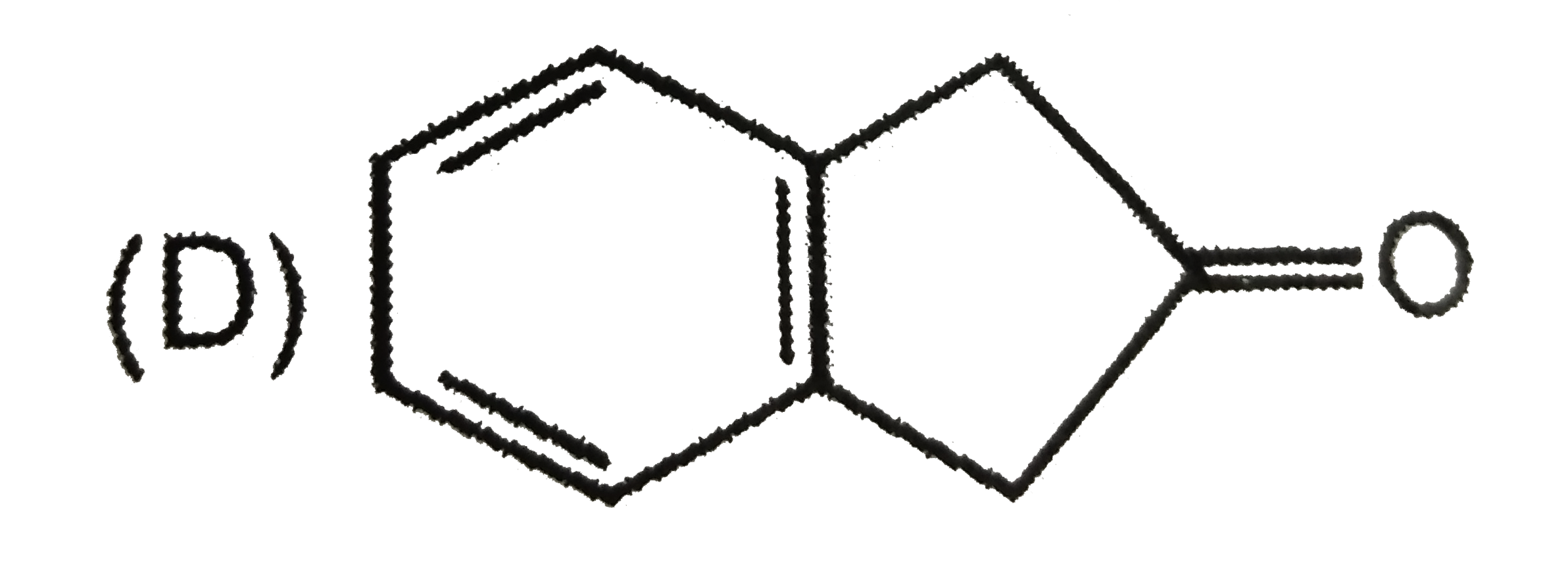
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise PART- IV|13 VideosCARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise Exercise-3|1 VideosCARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise Exercise-2 Part-I|1 VideosBIOMOLECULES & POLYMER
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Biomolecules & Polymer)|34 VideosCHEMICAL KINETICS
RESONANCE ENGLISH|Exercise PHYSICAL CHEMITRY (CHEMICAL KNIETICS & RADIOACTIVITY)|49 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID -Part-I
- Match the reactions in Column I with appropriate types of steps/reacti...
Text Solution
|
- The number of aldol reaction(s) that occurs in the given transformatio...
Text Solution
|
- In the following reaction sequence, the compound J is an interemedia...
Text Solution
|
- In the following reaction sequence, the compound J is an interemedia...
Text Solution
|
- After completion of the reactions (I and II), the organic compounds in...
Text Solution
|
- Among P,q,R and S, the aromatic compound(s) is /are:
Text Solution
|
- The major product of the following reaction is:
Text Solution
|
- In the following reactions, the product S is
Text Solution
|
- The major product of the following reactions sequence is
Text Solution
|
- Positive Tollen's test is observed for
Text Solution
|
- Compounds P and R upon ozonolysis produce Q and S, respectively. The m...
Text Solution
|
- There is a solution of p-hydroxybenzoic acid and p-amino benzoic acid ...
Text Solution
|
- Ph-underset(O)underset(||)(C)-NH(2) overset(POCl(3))to products is
Text Solution
|
- Identify the structure of the major products A,B, C and D formed in th...
Text Solution
|
- In the following reactions sequence, the correct structures of E,F and...
Text Solution
|
- In the reaction the structures of the product T is
Text Solution
|
- The carboxyl functional group(-COOH) is present in:
Text Solution
|
- The compound that undergoes decarboxylation most readily under mild co...
Text Solution
|
- The major product H of the given reaction sequence is : CH(3)-CH(2)-...
Text Solution
|
- The total number of carboxylic acid groups in the product P is
Text Solution
|