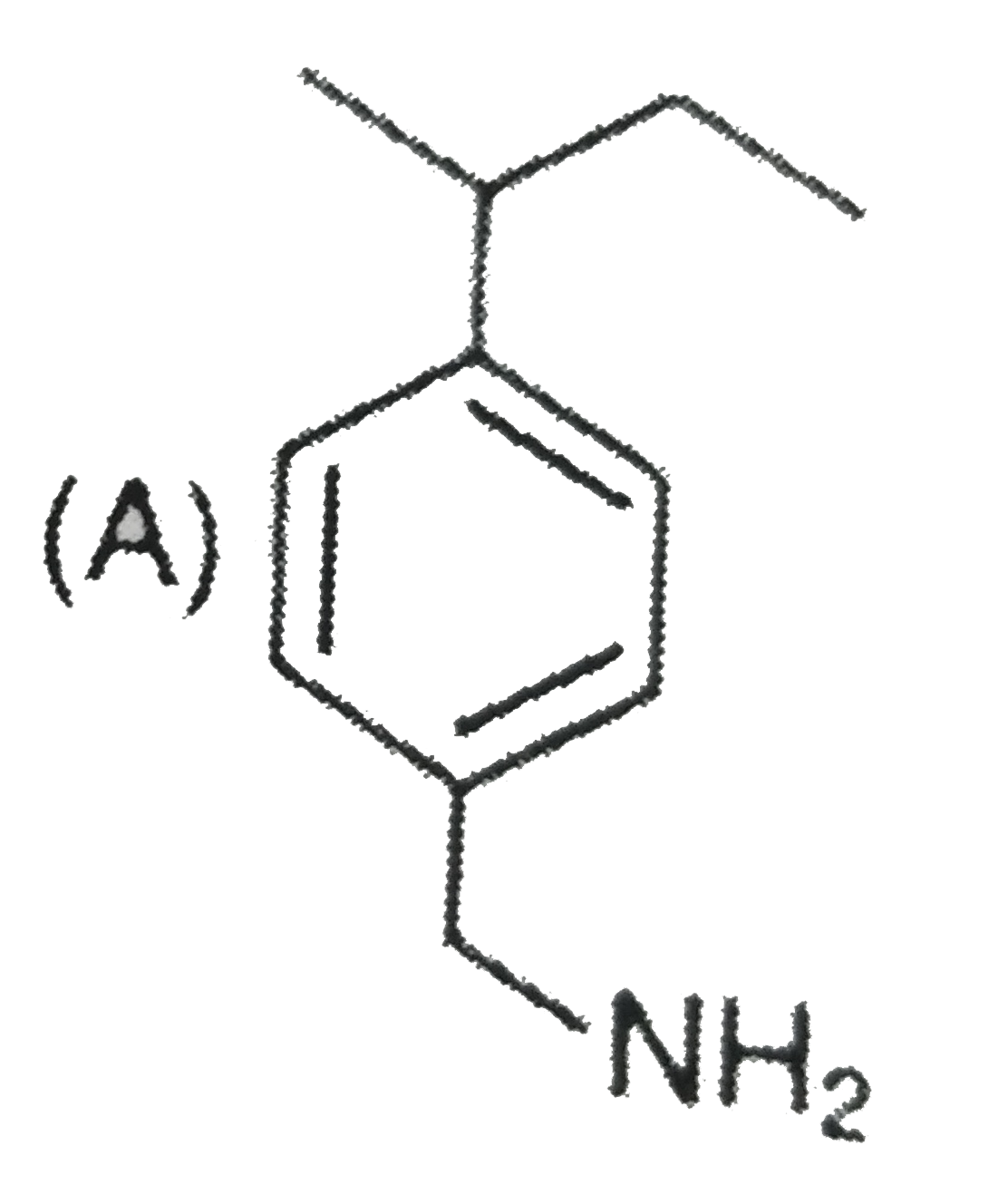
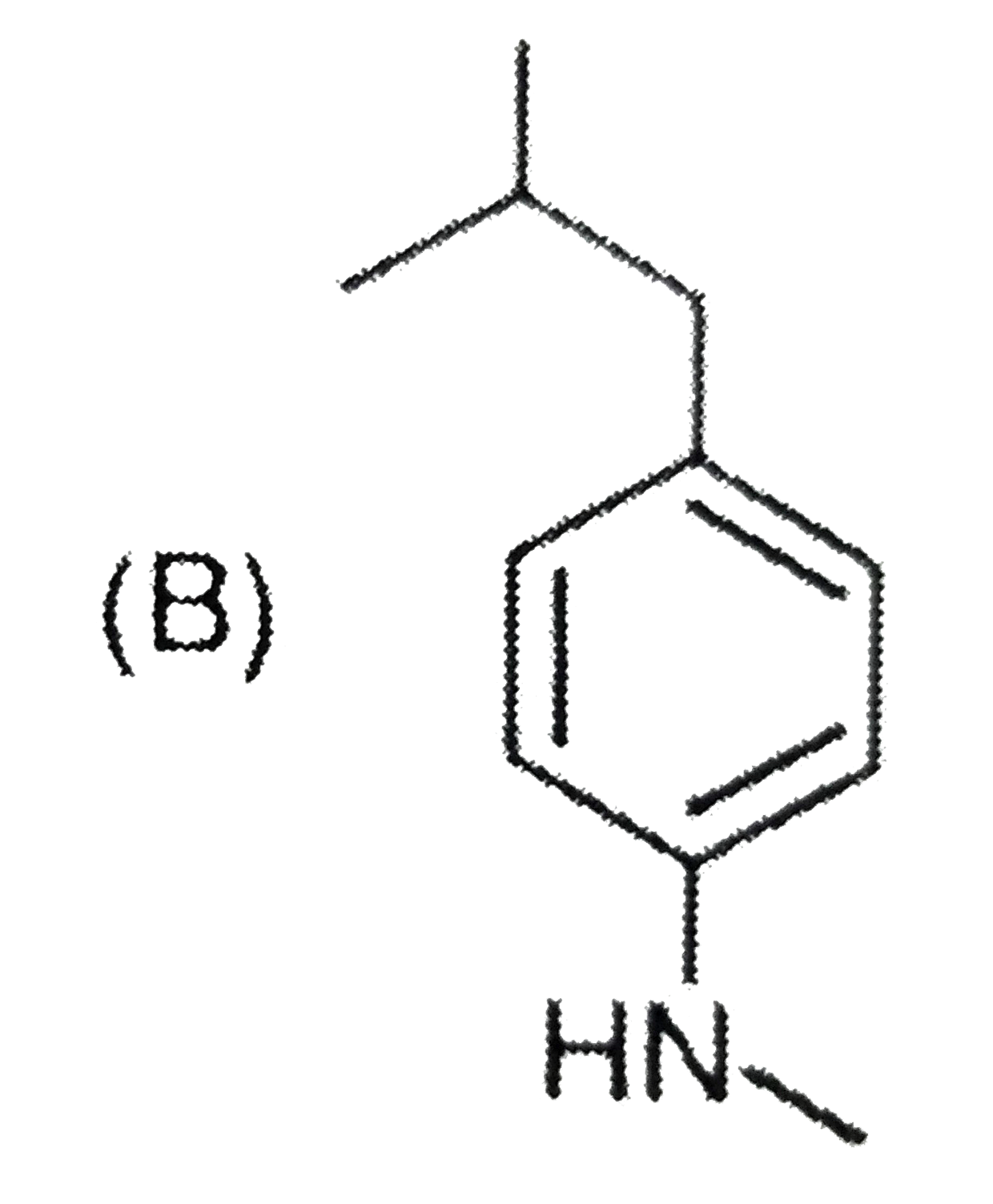
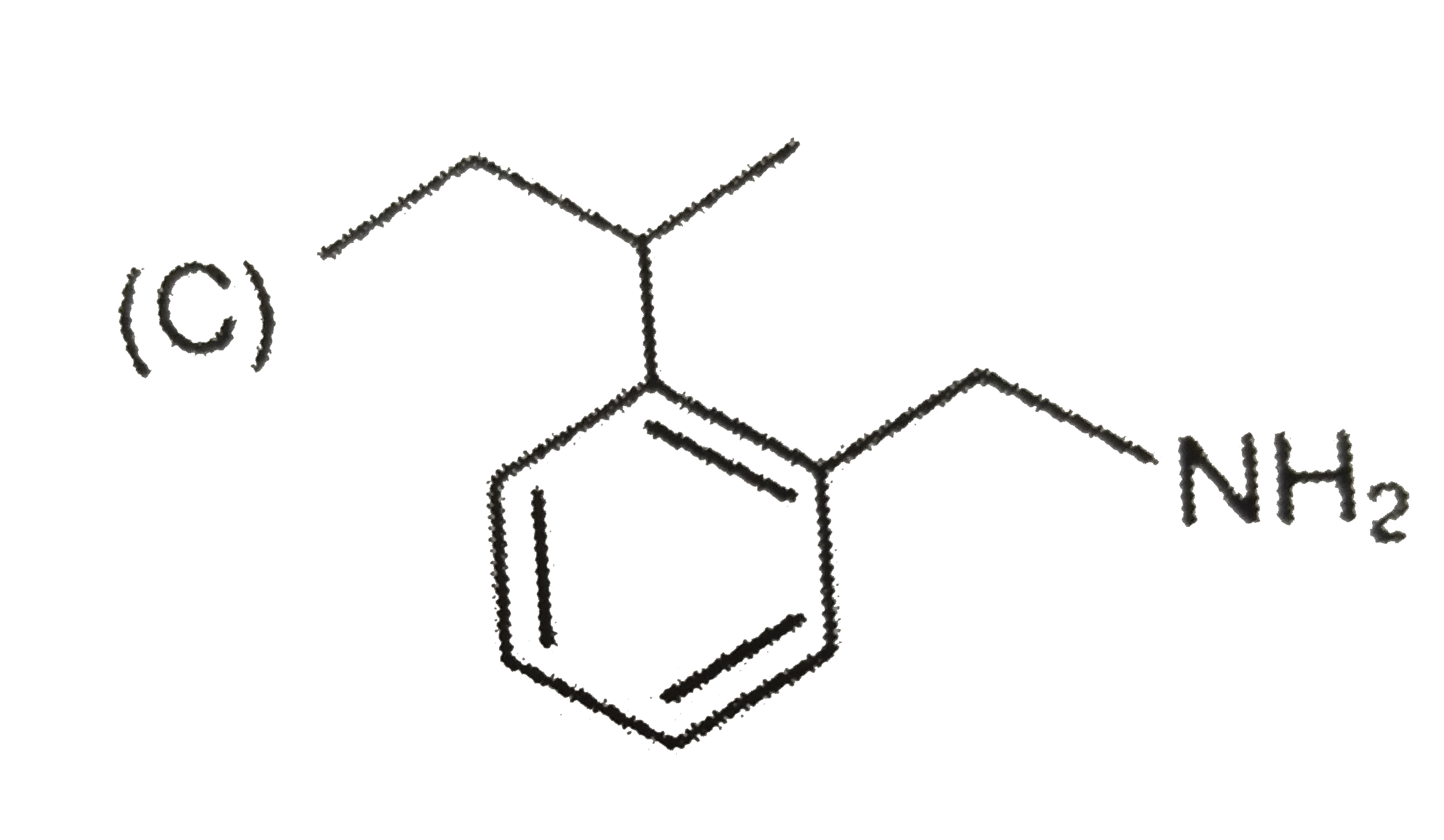
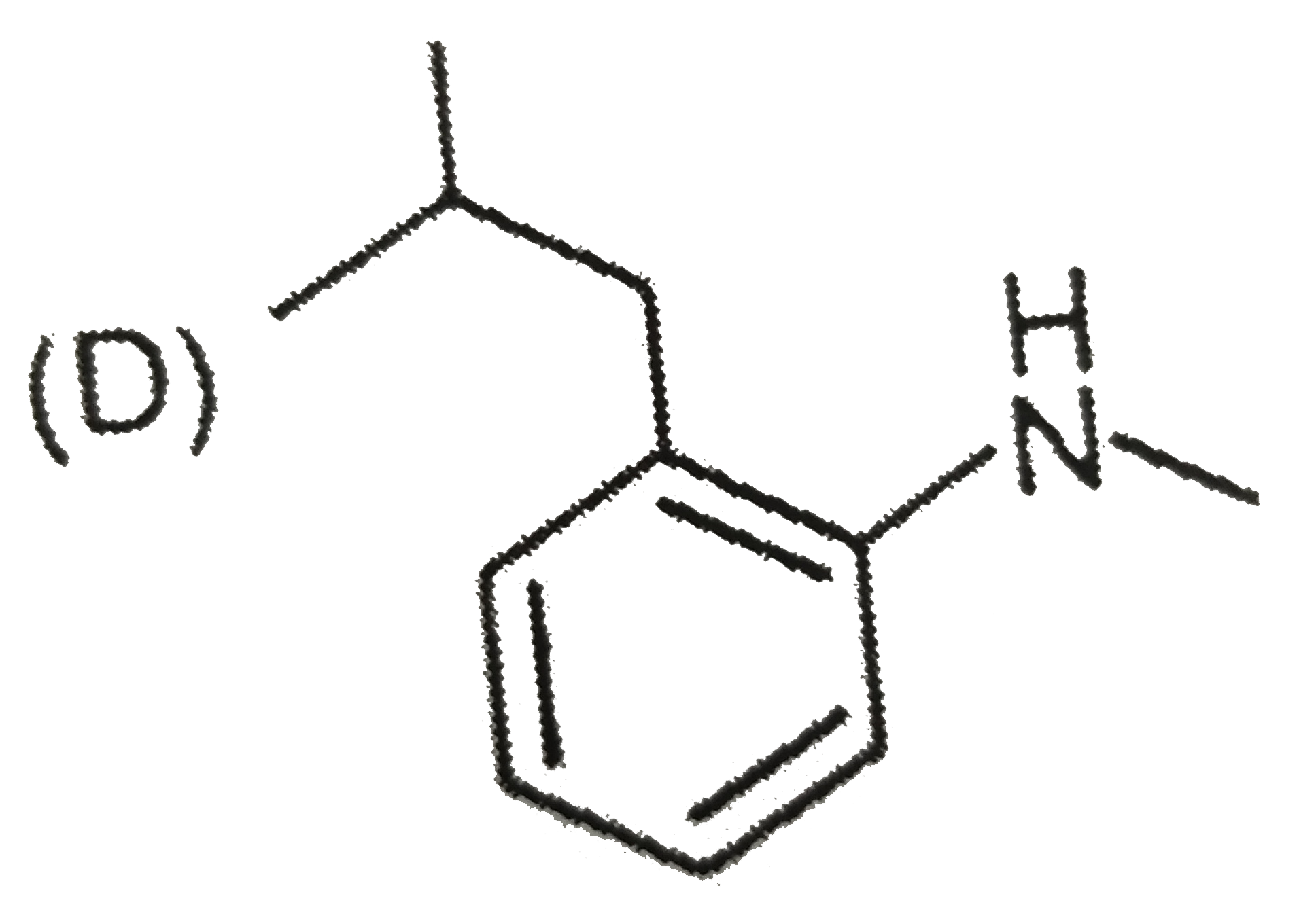
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise PART- IV|13 VideosCARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise Exercise-3|1 VideosCARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise Exercise-2 Part-I|1 VideosBIOMOLECULES & POLYMER
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Biomolecules & Polymer)|34 VideosCHEMICAL KINETICS
RESONANCE ENGLISH|Exercise PHYSICAL CHEMITRY (CHEMICAL KNIETICS & RADIOACTIVITY)|49 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID -Part-I
- Treatment of benzene with CO/HCl in the presence of anhydrous "AlCl"(3...
Text Solution
|
- An organic acid P (C(11)H(12)O(2)) can easily be oxidized to a dibasic...
Text Solution
|
- An organic acid P (C(11)H(12)O(2)) can easily be oxidized to a dibasi...
Text Solution
|
- Which of the following will be products of following reaction ?
Text Solution
|
- Under Wolff-Kishner reduction conditions, the conversion which may be ...
Text Solution
|
- An organic compound having molecular formula C(3)H(6)O does not react ...
Text Solution
|
- In the given reaction sequence Ph-CH(2)-CHO overset(NaOH)to overset(...
Text Solution
|
- Among the following compounds, which will react with acetone to give a...
Text Solution
|
- Consider following intramolecular aldol condensation reaction X c...
Text Solution
|
- The compound X on treatment with acidified K(2)Cr(2)O(7) gives compoun...
Text Solution
|
- What is the final products (B) of this reaction sequences ?
Text Solution
|
- Cannizzaro reaction does not take place with
Text Solution
|
- Which of the following does not give haloform reaction:
Text Solution
|
- Synthesis of an ester involves the reaction of alcohols with
Text Solution
|
- When phenyl magnesium bromide reacts with t-butanol the product would ...
Text Solution
|
- Products A of the above reactions is
Text Solution
|
- In the reaction sequence CH(3)-overset(O)overset(||)(C)-H-underset(...
Text Solution
|
- on reductive ozonolysis yeilds
Text Solution
|
- A cyanohydrin of a compound X on hydrolysis gives lactic acid. The X i...
Text Solution
|
- Which of the following will not undergo aldol condensation?
Text Solution
|