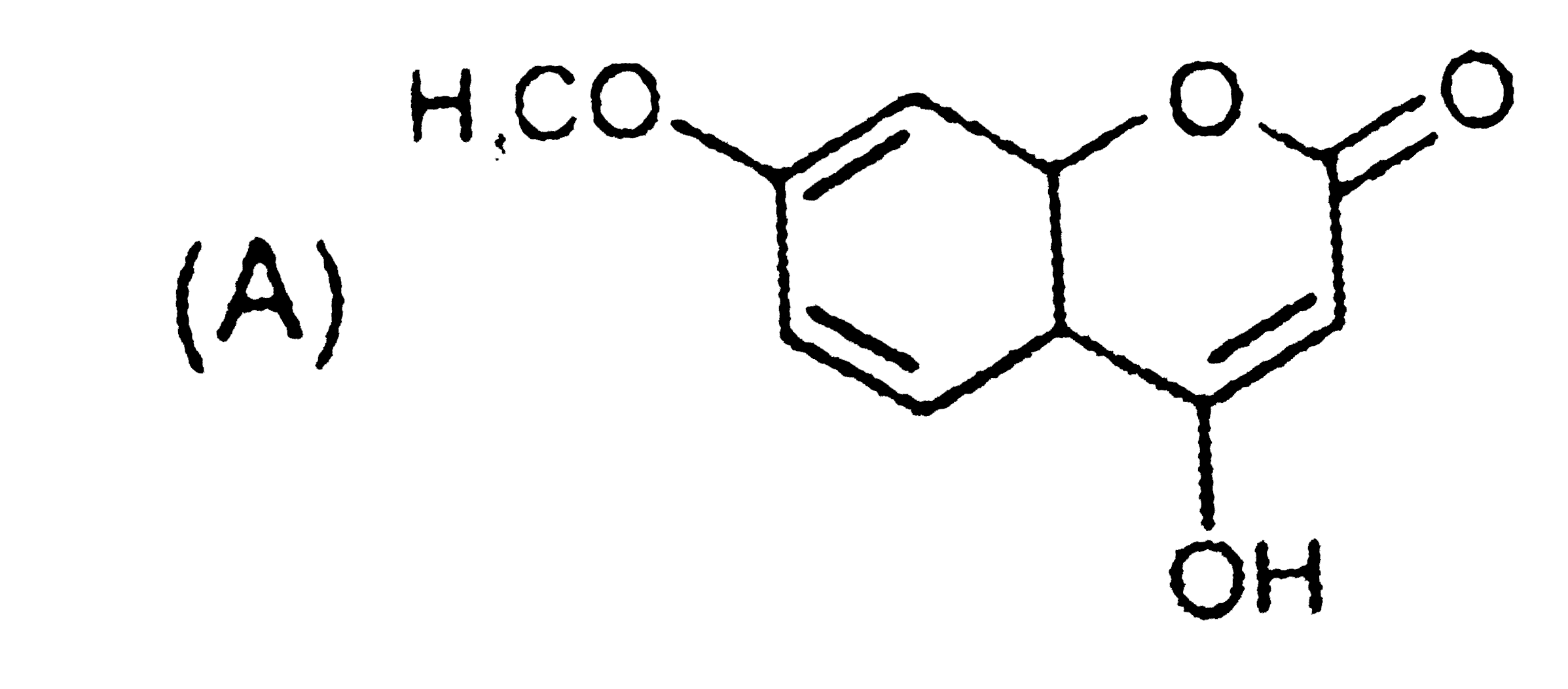
A
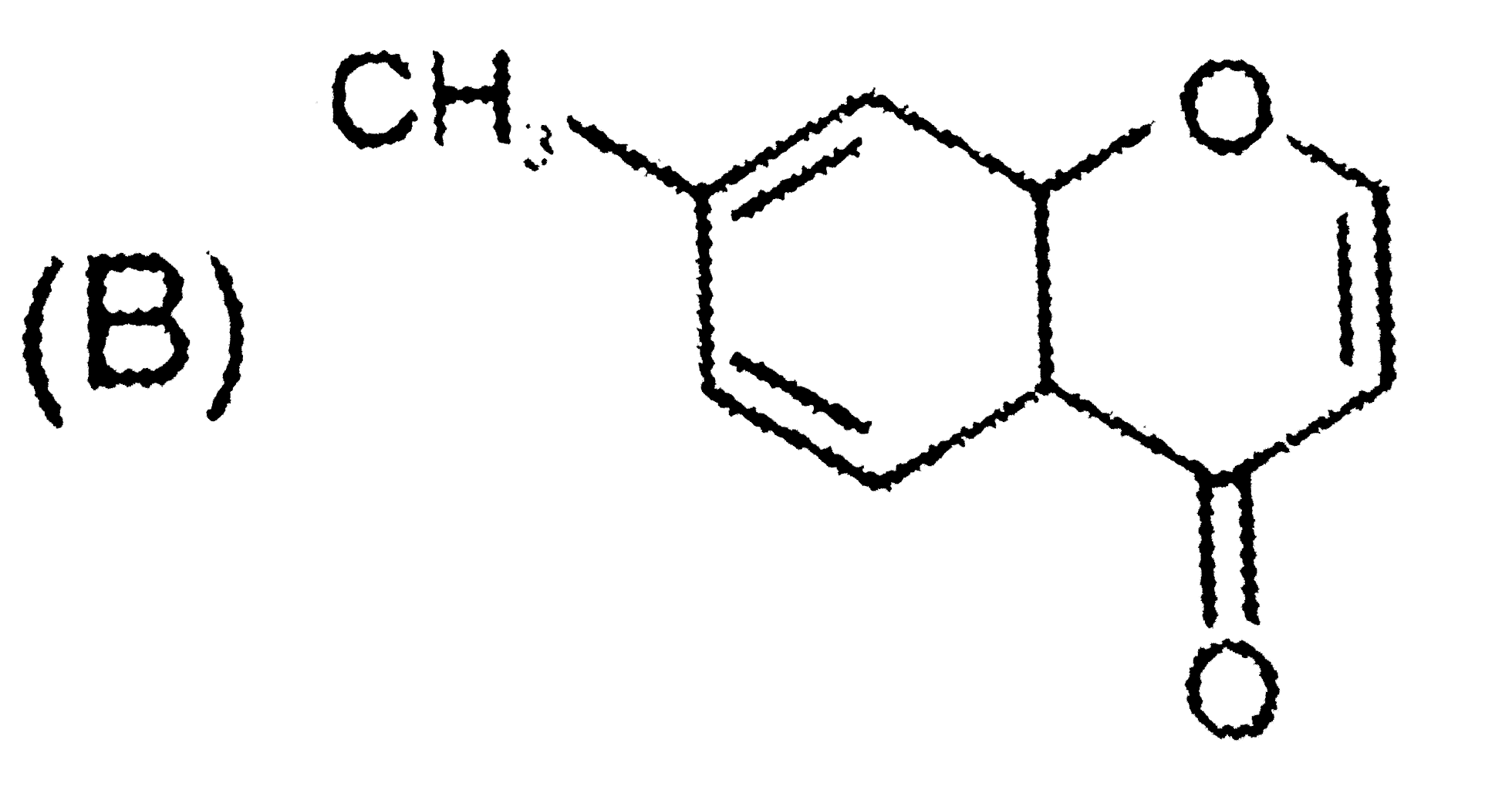
B
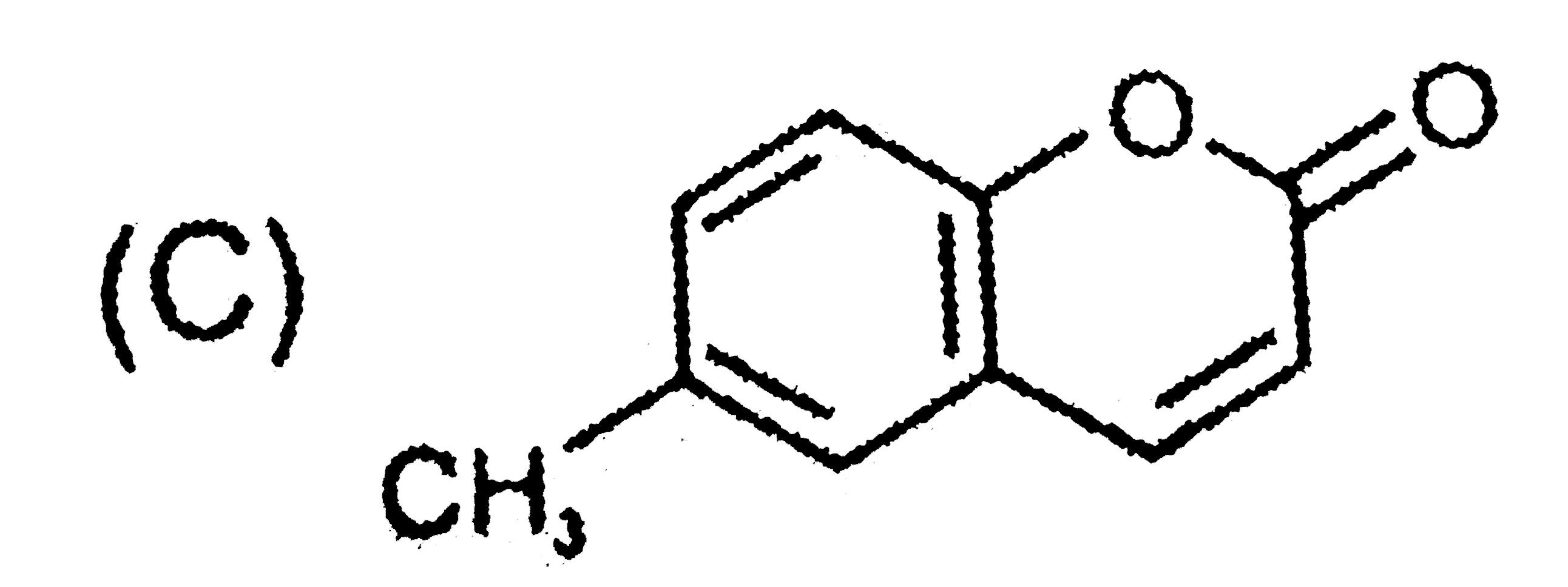
C
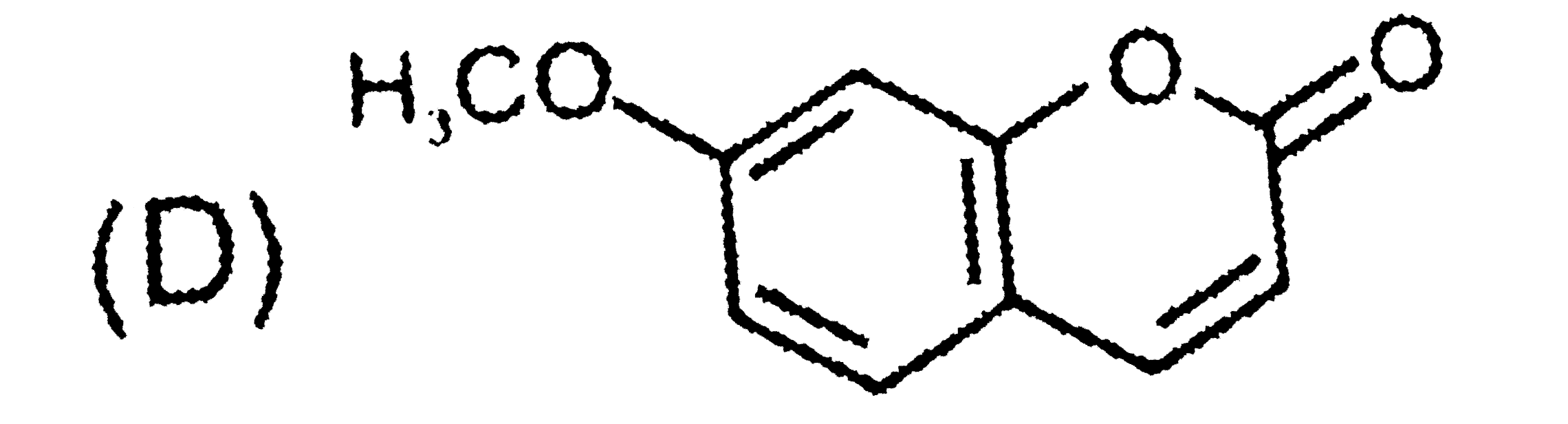
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise Part -III|31 VideosCARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise Part -IV|23 VideosCARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise APSP|1 VideosBIOMOLECULES & POLYMER
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Biomolecules & Polymer)|34 VideosCHEMICAL KINETICS
RESONANCE ENGLISH|Exercise PHYSICAL CHEMITRY (CHEMICAL KNIETICS & RADIOACTIVITY)|49 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID -Part -II
- The pair of the equimolar compound that would be give a single condens...
Text Solution
|
- The main product X formed in the following reaction is
Text Solution
|
- An organic compound ("X") is a disubstituted benzene containing 77.8% ...
Text Solution
|
- Compound "X" undergoes the following sequences of reactions to form "Y...
Text Solution
|
- The product ("X") of the following sequences of reactions is
Text Solution
|
- Three isometic compounds M,N and P (C(5)H(10)O) give the following tes...
Text Solution
|
- Give the structure of 4-Hydroxy-4-methylpentanal
Text Solution
|
- Compound "X" in the following reaction is
Text Solution
|
- Compound "X" in the following reaction is
Text Solution
|
- Compound 'P' that undergoes the sequence of reactions given below to g...
Text Solution
|
- The major product of the following reaction is
Text Solution
|
- The reaction , RCH(2)CH(2)COOH overset("Red P") underset(Br(2)) to R ...
Text Solution
|
- Formic acid and acetic acid can be distinguished with
Text Solution
|
- One of the product formed in the electrolysis of sodium salt of succin...
Text Solution
|
- The compound that gives a lactone on heating is
Text Solution
|
- The compound that undergoes decarboxylation most readily under mild co...
Text Solution
|
- The most unstable compounds fo the following are :
Text Solution
|
- The product obtained when propanamide is distilled with phosphorous pe...
Text Solution
|
- The product B of the following sequence of reactions is C(6)H(5)CONH...
Text Solution
|
- What is the major product obtained from the following reactions ? HO...
Text Solution
|