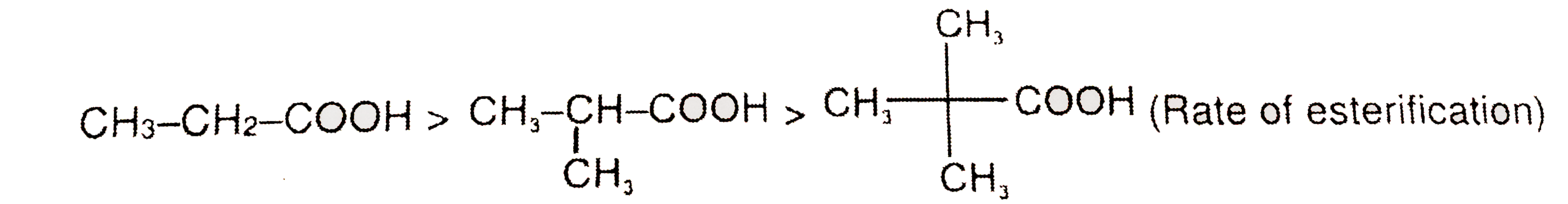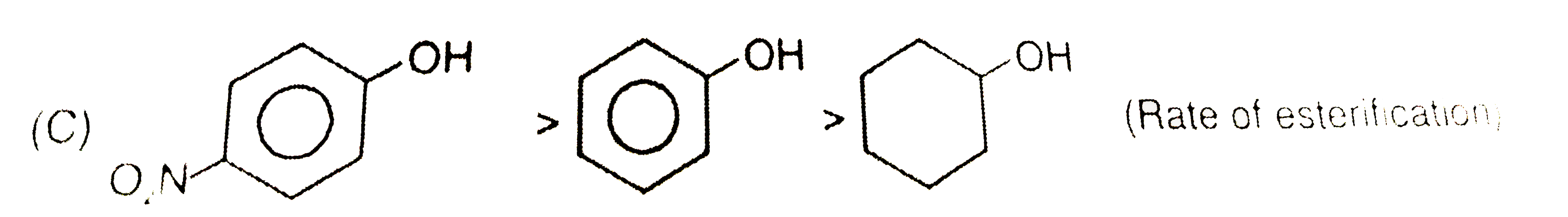A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise Part -IV|23 VideosCARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise Part -II|39 VideosBIOMOLECULES & POLYMER
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Biomolecules & Polymer)|34 VideosCHEMICAL KINETICS
RESONANCE ENGLISH|Exercise PHYSICAL CHEMITRY (CHEMICAL KNIETICS & RADIOACTIVITY)|49 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID -Part -III
- Product of following reactions is
Text Solution
|
- 2-Methychlcyclohexanone is allowed to react with matachloroperbenzoic ...
Text Solution
|
- Which optically active compound on reduction with LiAlH(4) will give o...
Text Solution
|
- The reaction,RCOOR'+R''OH (excess) overset(H^(+)or OH^(-))to RCOOR''+R...
Text Solution
|
Text Solution
|
- Match the product of column -II with the reaction given in column -I
Text Solution
|
- How many compounds out of the following are more reactive than ethyl a...
Text Solution
|
- The product of the following reactions is optically inactive and exist...
Text Solution
|
- Ph-overset(O)overset(||)(C)-CH(3)+underset("(excess)")(CH(2)=O) overse...
Text Solution
|
- Which of correct against property mentioned ?
Text Solution
|
- The following conversion is/are possible by Ph-CH(2)-CH=O to overset...
Text Solution
|
- Observe the esterification mechanisms for primary and teriary alcohols...
Text Solution
|
- Observe the esterification mechanisms for primary and teriary alcohols...
Text Solution
|
- Observe the esterification mechanisms for primary and teriary alcohols...
Text Solution
|
- Observe the following sequence of reaction and answer the questions ba...
Text Solution
|
- Observe the following sequence of reaction and answer the questions ba...
Text Solution
|
- Observe the following sequence of reaction and answer the questions ba...
Text Solution
|
- Ester having alpha-hydrogen on treatement with a strong base eg. C(2)H...
Text Solution
|
- Ester having alpha-hydrogen on treatement with a strong base eg. C(2)H...
Text Solution
|
- Ester having alpha-hydrogen on treatement with a strong base eg. C(2)H...
Text Solution
|