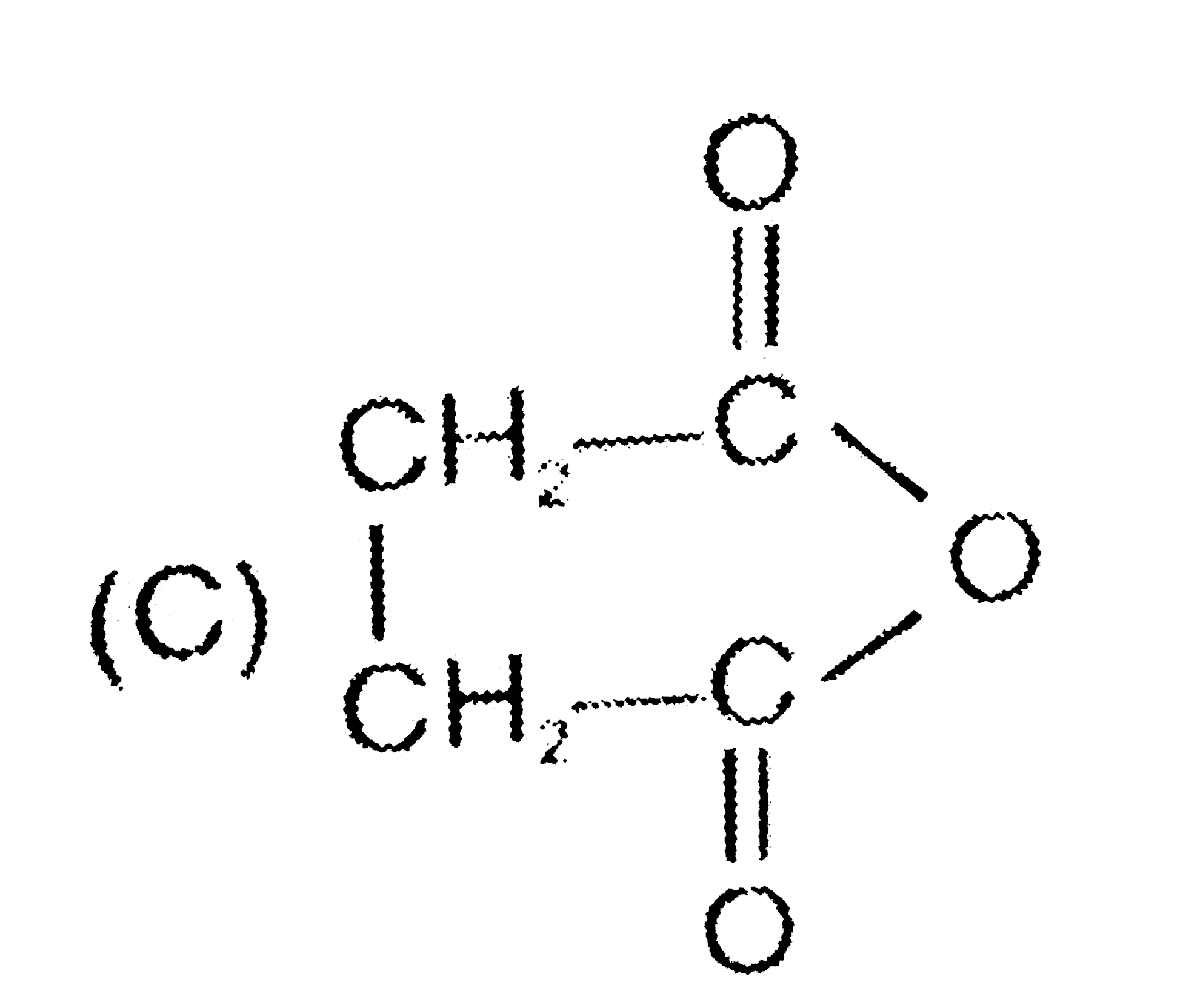
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID
RESONANCE ENGLISH|Exercise Part -III|31 VideosBIOMOLECULES & POLYMER
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Biomolecules & Polymer)|34 VideosCHEMICAL KINETICS
RESONANCE ENGLISH|Exercise PHYSICAL CHEMITRY (CHEMICAL KNIETICS & RADIOACTIVITY)|49 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID -Part -IV
- Which of the following reaction representes incorrect product
Text Solution
|
- In this reaction the product (E) is
Text Solution
|
- {:(CH(2)-CONH(2)),("|"),(CH(2)-CONH(2)):} overset(P(2)O(5))underset(De...
Text Solution
|
- The reaction between Benzaldehyde and methyl amine takes place in a ve...
Text Solution
|
- In the given reaction Product (P) will be
Text Solution
|
- Which of the following reaction will give beta-keto aldehyde as the fi...
Text Solution
|
- In the given reaction sequence Product (y) will be
Text Solution
|
- Compound (X) C(6)H(5)O gives yellow coloured ppt with 2,4 DNP but does...
Text Solution
|
- Product The correct statements about products is/are
Text Solution
|
- Which of the following releations is correct ?
Text Solution
|
- In which of the following reactions correct major product has be menti...
Text Solution
|
- Which of the following methods would not serve to prepare 1-phenylprop...
Text Solution
|
- How many of the following will give 2,4-DNP test. 1.CH(3)CH=O " "...
Text Solution
|
- Structure of A is
Text Solution
|
- Structure of (B) and (C) differentiated by
Text Solution
|
- structure of glucose is
Text Solution
|
- Give the Reaction of ethylalcohol with Boric Acid.
Text Solution
|
- Perkin reaction is the condensation reaction between aromatic aldehyde...
Text Solution
|
- Perkin reaction is the condensation reaction between aromatic aldehyde...
Text Solution
|
- Match the column
Text Solution
|