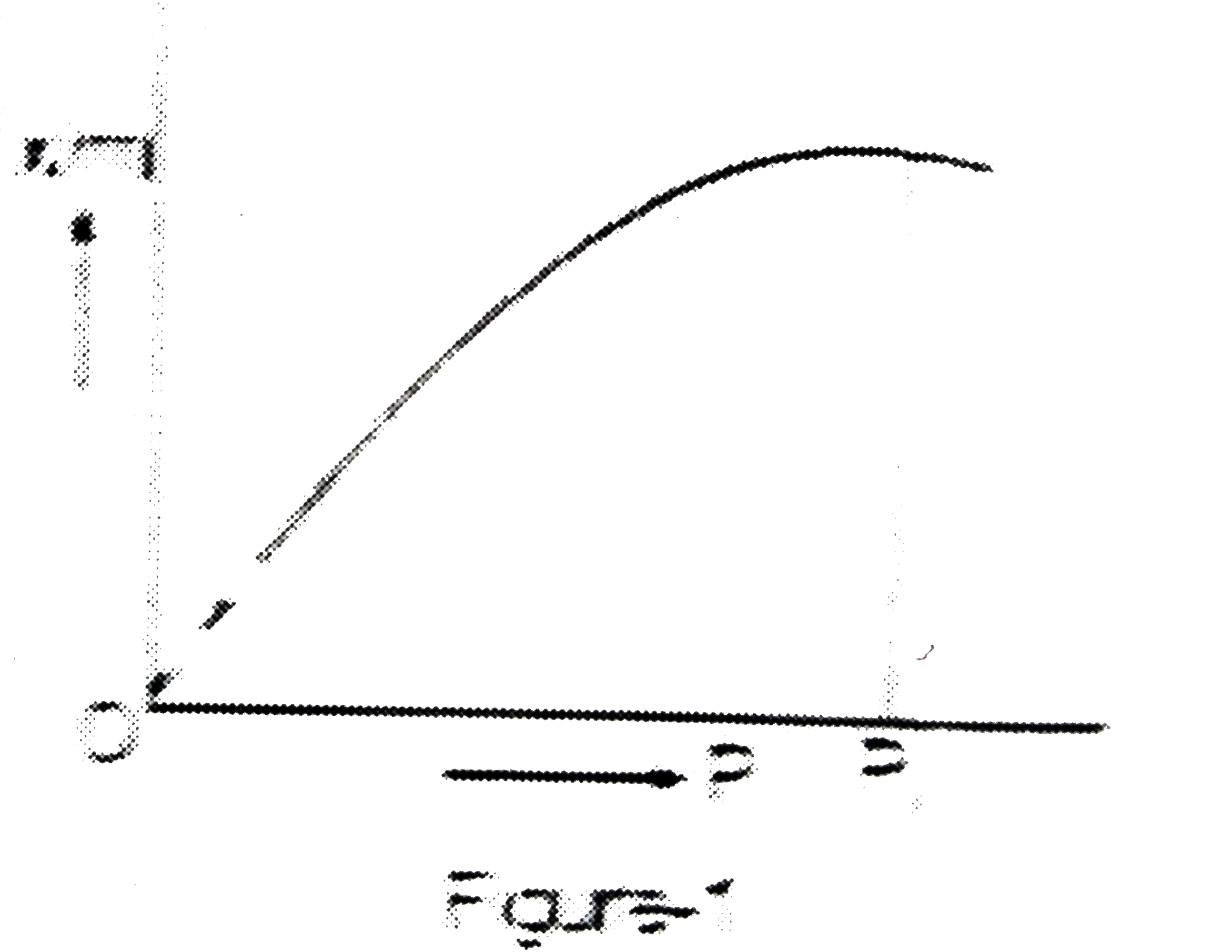
Text Solution
Verified by Experts
Topper's Solved these Questions
SURFACE CHEMISTRY
RESONANCE ENGLISH|Exercise Section (B)|4 VideosSURFACE CHEMISTRY
RESONANCE ENGLISH|Exercise Section (C)|7 VideosSURFACE CHEMISTRY
RESONANCE ENGLISH|Exercise Solved Problems|11 VideosSTRUCTURAL IDENTIFICATION
RESONANCE ENGLISH|Exercise Advanced level Problems (Part-III)|12 VideosTEST PAPERS
RESONANCE ENGLISH|Exercise FST-3|30 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-SURFACE CHEMISTRY-Exercise-1, Part-I, Section (A)
- Why is adsorption always exothermic ?
Text Solution
|
- What is the difference between physisorption and chemisorption?
Text Solution
|
- What are the factors which influence the adsorption of a gas on a soli...
Text Solution
|
- What is an adsorption isotherm ?
Text Solution
|
- What do you understand by activation of adsorbent? How is it achieved ...
Text Solution
|
- Which will be adsorbed more readily on the surface of charcoal and why...
Text Solution
|
- In an Adsorption experiment a graph between log x/m versus log P was f...
Text Solution
|
- One gram of charcoal adsorbs 100ml of 0.5M acetic acid to form a monol...
Text Solution
|
- What role does adsorption play in heterogeneous catalysis?
Text Solution
|
- How many grams of gas would be adsobed per gram of a substance at 8 at...
Text Solution
|
- 10 mg of an adsorbate gets adsorbed on a surface. This cause the relea...
Text Solution
|