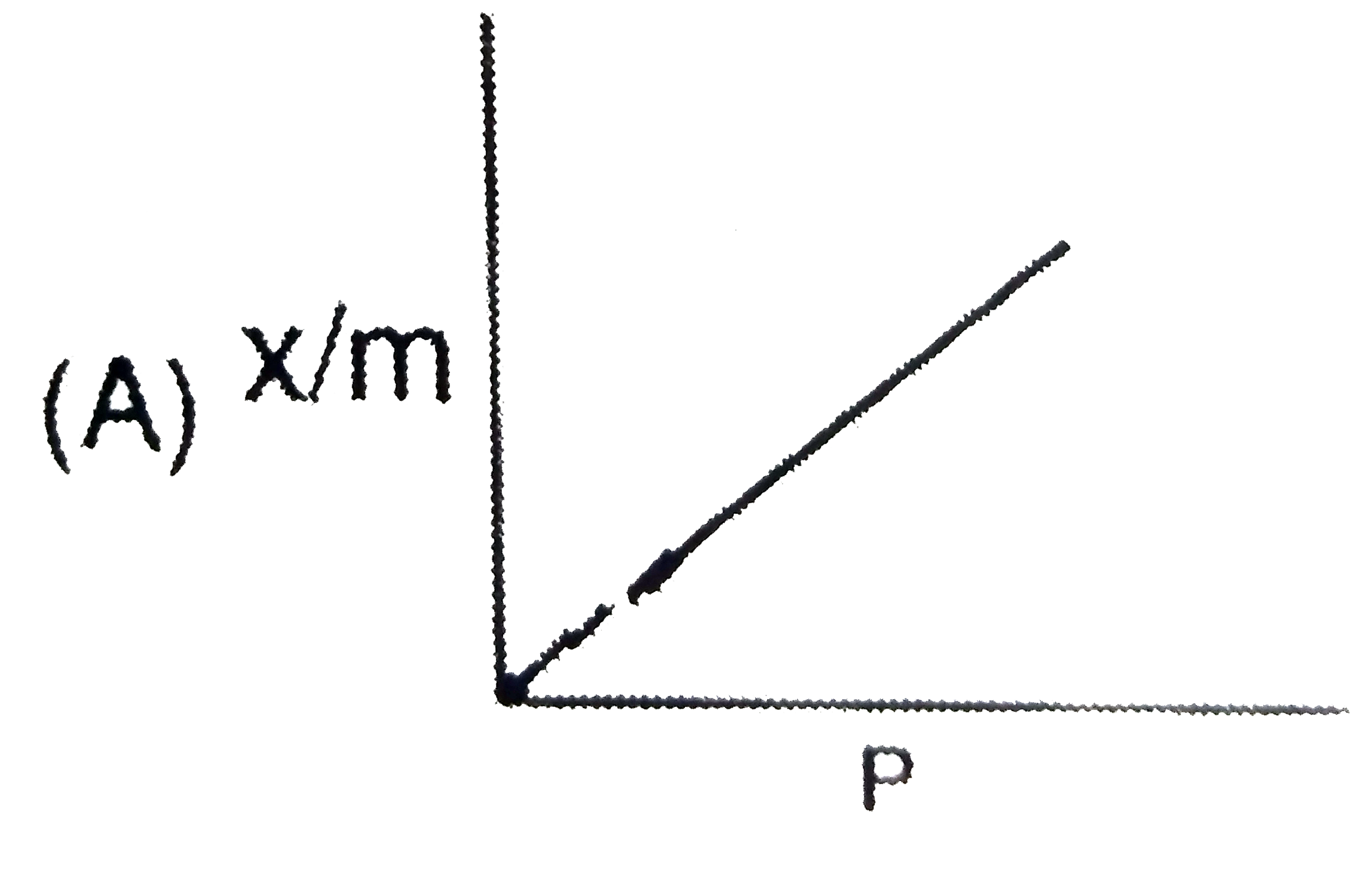
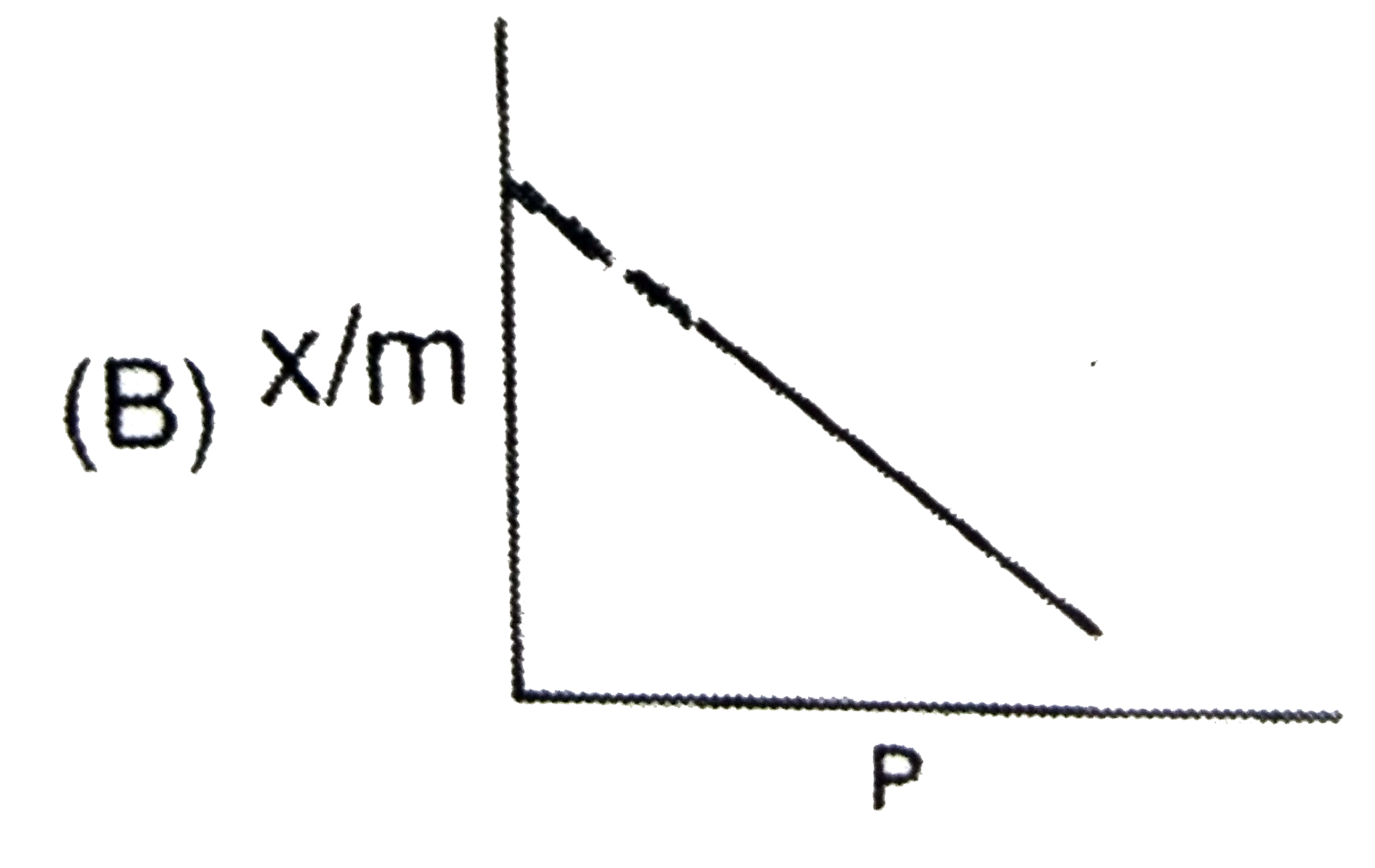
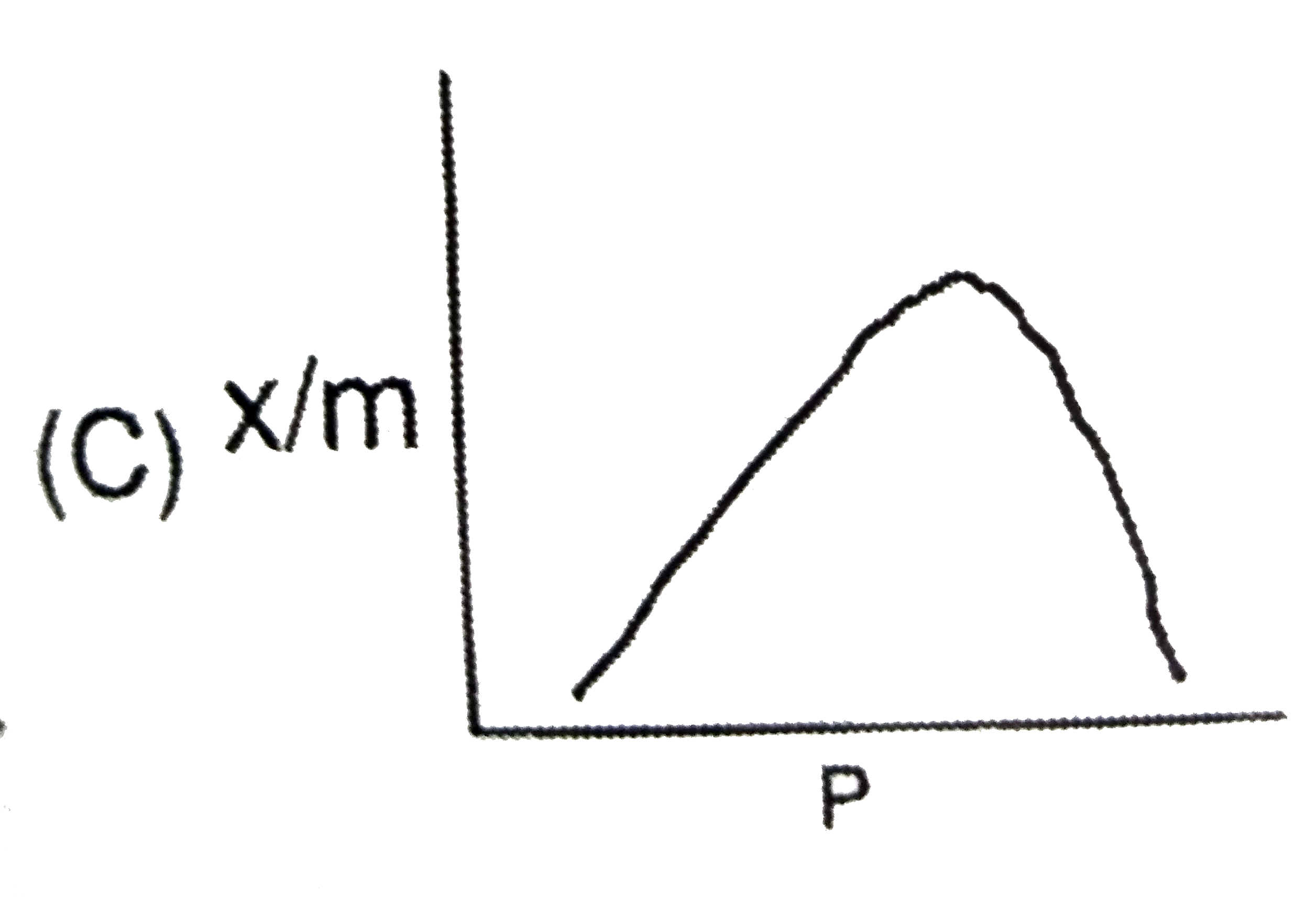
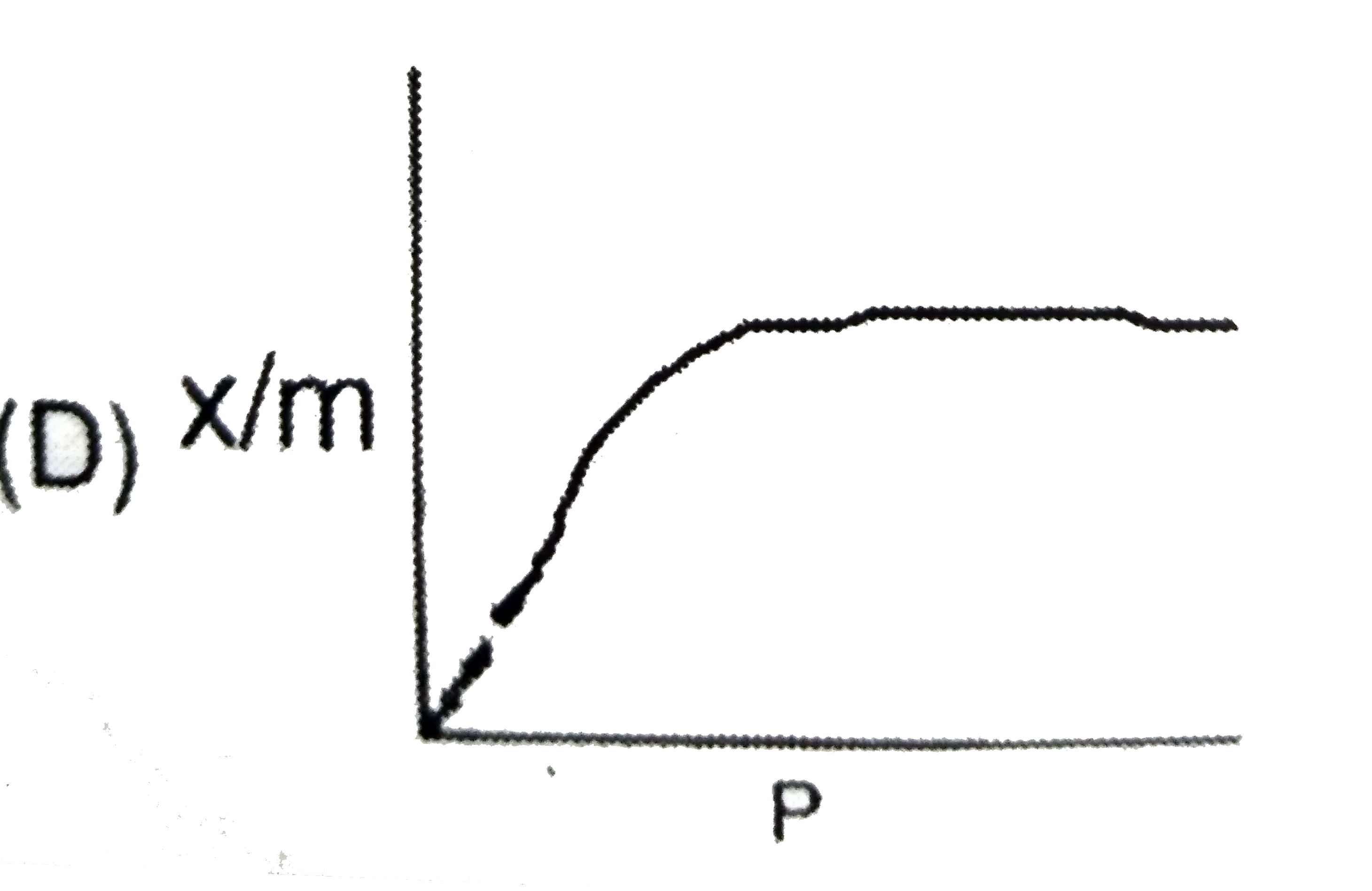
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
SURFACE CHEMISTRY
RESONANCE ENGLISH|Exercise Part - III : High Level Problems|2 VideosSURFACE CHEMISTRY
RESONANCE ENGLISH|Exercise Only One Option Correct Type|7 VideosSURFACE CHEMISTRY
RESONANCE ENGLISH|Exercise Part - I Practice Test - 1 (IIT - JEE (Main Pattern))|29 VideosSTRUCTURAL IDENTIFICATION
RESONANCE ENGLISH|Exercise Advanced level Problems (Part-III)|12 VideosTEST PAPERS
RESONANCE ENGLISH|Exercise FST-3|30 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-SURFACE CHEMISTRY-Part - II : National Standard Examination in Chemistry Stage - 1
- Soaps essentially form a colloidal solution in water and remove the gr...
Text Solution
|
- Swimming for a long time in salt water makes the skin of one's finger ...
Text Solution
|
- Tyndall effect in colloidal solution is due to .
Text Solution
|
- Ferric chloride is applied to stop bleeding because:
Text Solution
|
- A catalyst is a substance which :
Text Solution
|
- In nature, ammonia is synthesisd by nitrifying bacteria using enzymes ...
Text Solution
|
- A catalyst increases the
Text Solution
|
- Soaps essentially form a colloidal solution in water and remove the gr...
Text Solution
|
- Smoke is an example of
Text Solution
|
- A catalyst speeds up a chemical reraction by
Text Solution
|
- The plot representing Langmuir's adsorption isotherm is :
Text Solution
|
- Frendlich adsorption isotherms are properly represented as in
Text Solution
|
- A gold sol is prepared by :
Text Solution
|
- Effective electrolyte to cause the flocculation of a negatively charge...
Text Solution
|
- A catalyst is a substance which :
Text Solution
|
- Which of the following reaction parameters will change due to addition...
Text Solution
|
- In electrophoresis,
Text Solution
|
- 100 mL of 0.3 M acetic acid is shaken with 0.8 g wood charcoal. The fi...
Text Solution
|
- The mass of argon adsorbed per unit mass of carbon surface is plotted ...
Text Solution
|
- The equation of state of some gases can be expressed as (p+(a)/(V^(2...
Text Solution
|