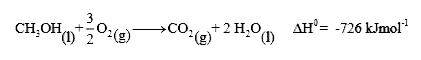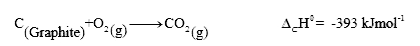Similar Questions
Explore conceptually related problems
Recommended Questions
- Calculate the standard enthalpy of formation of liquid methanol from t...
Text Solution
|
- Calculate the standard deviation from the following data:
Text Solution
|
- Calculate standard deviation from the following data:
Text Solution
|
- Calculate mean and standard deviation from the following data:
Text Solution
|
- The approxiamte standard enthalpies of formation of methanol and octan...
Text Solution
|
- निम्न विकल्पों में से वो अभिक्रिया (अभिक्रियाएँ) जिसकी (जिनकी) मानक अभ...
Text Solution
|
- Standard enthalpy of formation is zero for ........... from following
Text Solution
|
- Explain the step for calculation of standard enthalpy of reaction from...
Text Solution
|
- If the standard enthalpy of formation of methanol is -238.9 kJ mol^(-1...
Text Solution
|