Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ORGANIC COMPOUNDS CONTAINING NITROGEN-EXERCISE
- Give a chemical test to distinguish between ethylamine and aniline.
Text Solution
|
- What happens when acetamide is heated with phosphorus pentoxide and th...
Text Solution
|
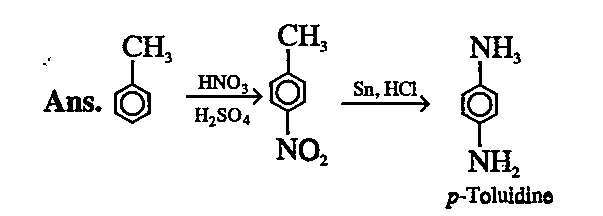
- How will you prepare p-toluidine from toluene?
Text Solution
|
- How is m-nitroaniline obtained from nitrobenzene?
Text Solution
|
- Sulphanilic acid has acidic as well as basic groups, but it is soluble...
Text Solution
|
- Arrange the following in increasing order of their basic strength in a...
Text Solution
|
- What is the role of HNO(3) in the nitrating mixture used for nitration...
Text Solution
|
- Why is benzene diazonium chloride not stored and used immediately afte...
Text Solution
|
- Why aniline is soluble in aq. HCl?
Text Solution
|
- Tert-butylamine cannot be prepared by the action of NH(3) on tert-buty...
Text Solution
|
- Explain : 2-aminoethanoic acid exists as a dipolar ion as does p-amino...
Text Solution
|
- Amines are basic substances while amides are neutral.Explain.
Text Solution
|
- Aromatic amines are weaker bases than aliphatic amines. True / False
Text Solution
|
- Why ethylamine is a stronger base than methylamine?
Text Solution
|
- Why reactivity of -NH(2) group gets reduced in acetanilide?
Text Solution
|
- Alkylamines are stronger bases than arylamines.Explain.
Text Solution
|
- How would you convert methylamine into ethylamine?
Text Solution
|
- Why amines are more basic than the comparable alcohols ?
Text Solution
|
- Account for the following Methylamine in water reacts with ferric ch...
Text Solution
|
- It is difficult to prepare pure amines by ammonolysis of alkyl halides...
Text Solution
|