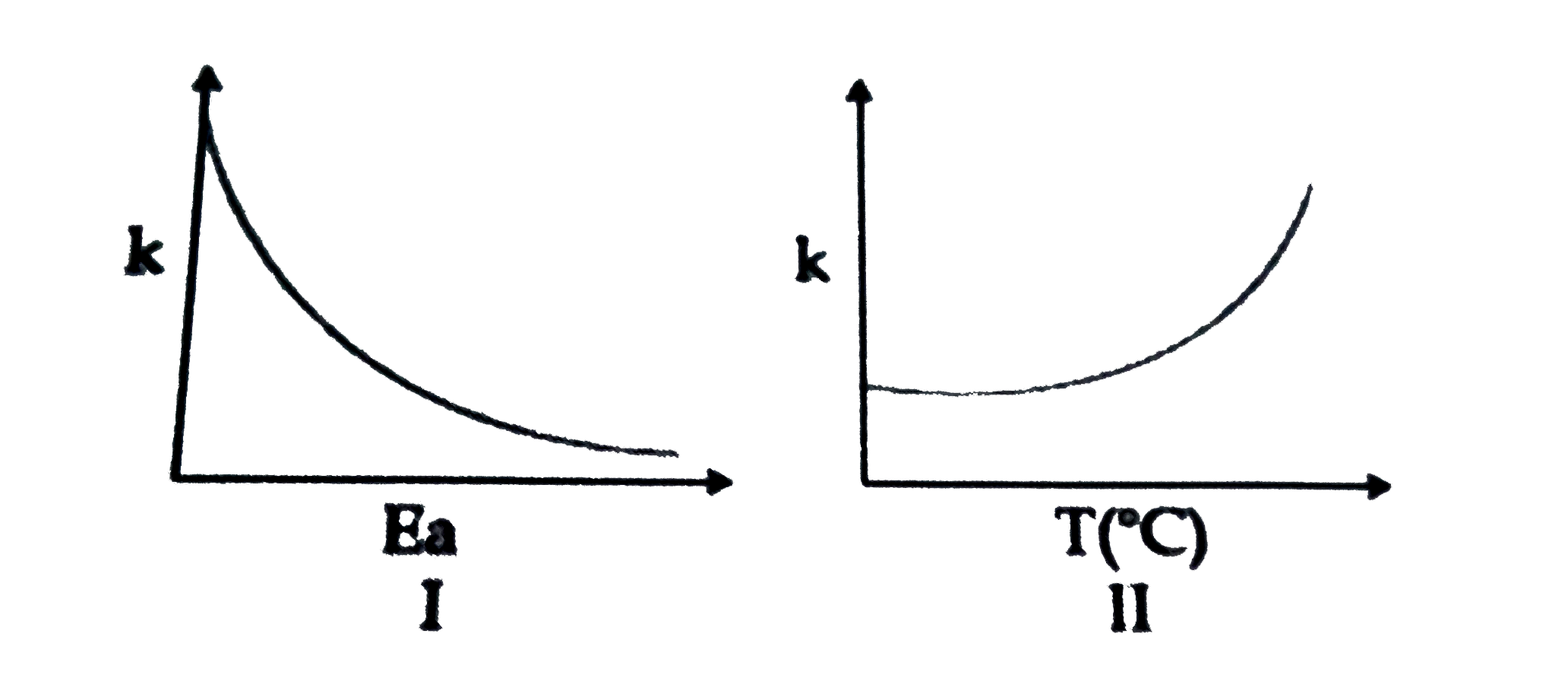
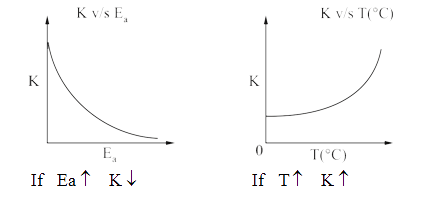
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-JEE MAIN REVISION TEST - 4 JEE - 2020-CHEMISTRY
- The major product ‘X’ formed in the following reaction is:
Text Solution
|
- A mixture of 100m mol of Ca(OH)(2) and 2g of sodium sulphate was dis...
Text Solution
|
- Consider the given plots for a reaction obeying Arrhenius equation (0^...
Text Solution
|
- The increasing order of the pKa values of the following compounds is:
Text Solution
|
- Which dicarboxylic acid in presence of a dehydrating agent is least re...
Text Solution
|
- Which of the graphs shown below does not represent the relationship be...
Text Solution
|
- The values of K(p)//K(c) for the following reactions at 300 K are, res...
Text Solution
|
- Wilkinson catalyst is :
Text Solution
|
- Which hydrogen in compound (E) is easily replaceable during brominatio...
Text Solution
|
- The decreasing order of ease of alkaline hydrolysis for the following ...
Text Solution
|
- The effect of lanthanoid contraction in the lanthanoid series of eleme...
Text Solution
|
- Which of the following is not an example of heterogeneous catalytic re...
Text Solution
|
- The major product of the following reaction is:
Text Solution
|
- Hall-Heroult's process is given by :
Text Solution
|
- The chemical nature of hydrogen peroxide is :
Text Solution
|
- The metal used for making X-ray tube window is :
Text Solution
|
- Consider the following reduction processes : Zn^(2+)+2e^(-)toZn(s), ...
Text Solution
|
- Water filled in two glasses A and B have BOD value of 10 and 20, respe...
Text Solution
|
- Which premitive unit cell has unequual edge lengths (anebnec) and all ...
Text Solution
|
- The major product of the following reaction is:
Text Solution
|