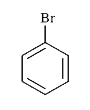
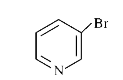
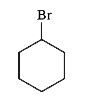
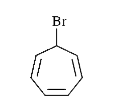
To determine which of the given compounds will produce a precipitate with AgNO₃, we need to analyze each compound's ability to form a precipitate with silver nitrate. A precipitate forms when a reaction leads to the formation of an insoluble compound. In this case, we are particularly interested in the formation of silver halides, such as AgBr.
### Step-by-Step Solution:
1. **Identify the Compounds**: We need to analyze the compounds provided in the options. Let's assume the compounds are XBr, YCl, ZI, and W (where W is a different compound).
2. **Reactions with AgNO₃**:
- **For XBr**:
- When XBr is reacted with AgNO₃, the reaction will be:
\[
\text{XBr} + \text{AgNO}_3 \rightarrow \text{AgBr} + \text{XN}O_3
\]
- AgBr is a yellow precipitate, indicating that XBr will produce a precipitate with AgNO₃.
- **For YCl**:
- The reaction will be:
\[
\text{YCl} + \text{AgNO}_3 \rightarrow \text{AgCl} + \text{YN}O_3
\]
- AgCl is a white precipitate, indicating that YCl will also produce a precipitate with AgNO₃.
- **For ZI**:
- The reaction will be:
\[
\text{ZI} + \text{AgNO}_3 \rightarrow \text{AgI} + \text{ZN}O_3
\]
- AgI is a yellow precipitate, indicating that ZI will also produce a precipitate with AgNO₃.
- **For W**:
- Depending on the nature of W, we need to analyze if it contains a halide that can react with AgNO₃. If W does not contain a halide, it will not produce a precipitate.
3. **Conclusion**:
- Based on the analysis, the compounds that will produce a precipitate with AgNO₃ are XBr, YCl, and ZI. The specific compound that produces the most stable precipitate, considering the stability of the resulting ions, is determined by the nature of the positive charge and resonance stability.
### Final Answer:
The compounds that will produce a precipitate with AgNO₃ are XBr, YCl, and ZI. The most stable compound among them is the one that shows resonance stabilization.