A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-JEE Main Revision Test-9 | JEE-2020 -CHEMISTRY SECTION 2
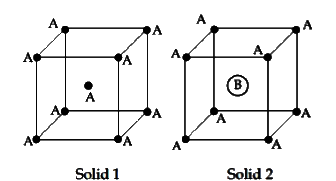
- Consider the bcc unit cells of the solids 1 and 2 with the position of...
Text Solution
|
- 5 moles of an ideal gas at 100 K are allowed to undergo reversible com...
Text Solution
|
- 0.27 g of a long chain fatty acid was dissolved in 100 cm^ 3 of ...
Text Solution
|
- How many isomers are possible C 7H (16) ?
Text Solution
|
- What is the value of spin only magnetic moment of anionic and cationic...
Text Solution
|
- If p is the momentum of the fastest electron ejected from a metal surf...
Text Solution
|