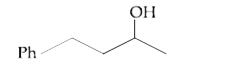
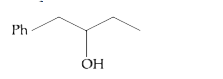
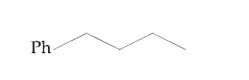
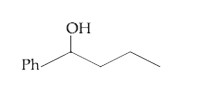
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-REVISION TEST-15 JEE - 2020-CHEMISTRY
- The compound used in the treatment of lead poisoning is :
Text Solution
|
- Among the following, the INCORRECT statement about colloids is :
Text Solution
|
- Assertion: Vinyl halides do not undergo substitution reaction. Reaso...
Text Solution
|
- In the following skew conformatioin of ethane, H'-C-C-H'' dihedral a...
Text Solution
|
- C-C bond length is maximum in
Text Solution
|
- A solution is prepared by dissolving 0.6 g of urea (molar mass =60 g"m...
Text Solution
|
- Heating of 2-chloro-1-phenylbutane with EtOK/EtOH gives X as the major...
Text Solution
|
- In comparison to B, Be has
Text Solution
|
- The decreasing order of electrical conductivity of the following aqueo...
Text Solution
|
- Benzene diazonium chloride on reaction with aniline in the presence of...
Text Solution
|
- Which one of the following is likely to give a precipitate with AgNO(3...
Text Solution
|
- The correct statement is :
Text Solution
|
- The temporary hardness of a water sample is due to compound X. Boiling...
Text Solution
|
- The INCORRECT statement is :
Text Solution
|
- Which of the given statements is INCORRECT about glycogen ?
Text Solution
|
- The molar solubility of Cd(OH)(2) is 1.84xx10^(-5)M in water. The expe...
Text Solution
|
- NO(2) required for a reaction is produced by the decomposition of N(2)...
Text Solution
|
- 25 g of an unknown hydrocarbon contains 24 g of carbon. 25 g of this h...
Text Solution
|
- The coordination numbers of Co in [Co(Cl)(en)(2)]Cl is. (en=ethane-1...
Text Solution
|
- The ratio of number of atoms present in a simple cubic, body centered ...
Text Solution
|