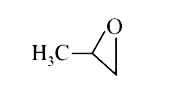
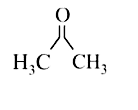A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-JEE MAIN REVISION TEST- 16-CHEMISTRY (SECTION 2)
- CH(3)-CH=CH(2)overset(Cl(2)//H(2)O)to The correct product is
Text Solution
|
- The molar solubility of Al(OH)(3) in 0.2 M NaOH solution is x xx 10^(...
Text Solution
|
- An ideal gas is allowed to expand from 1L to 10 L against a constant e...
Text Solution
|
- The sum of number of valence electrons and valency of an element with ...
Text Solution
|
- Glucose and Galactose are having identical configuration in all the po...
Text Solution
|
- If mass of 5 mole AB(2) is 125xx10^(-3)kg and mass of 10 mole A(2)B(2)...
Text Solution
|