A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-JEE MAIN REVISION TEST - 19-CHEMISTRY
- Which dicarboxylic acid in presence of a dehydrating agent is least re...
Text Solution
|
- The total number of isotopes of hydrogen and number of radioactive iso...
Text Solution
|
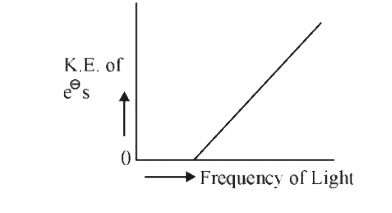
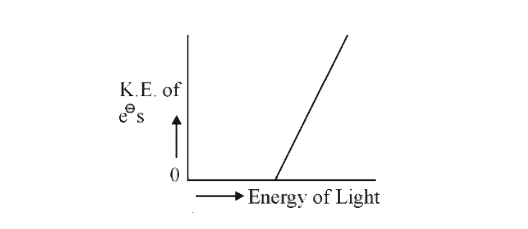
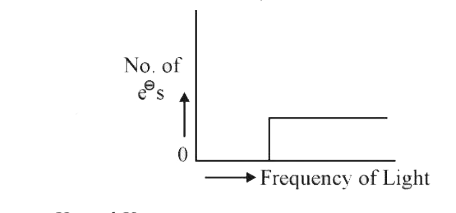
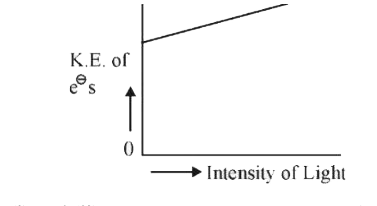
- Which of the graphs shown below does not represent the relationship be...
Text Solution
|
- K(1) and K(2) are equilibrium constants for reaction (i) and (ii) N(...
Text Solution
|
- Ziegler-Natta catalyst is
Text Solution
|
- Two pi and half sigma bonds are present in :
Text Solution
|
- The decreasing order of ease of alkaline hydrolysis for the following ...
Text Solution
|
- The effect of lanthanoid contraction in the lanthanoid series of eleme...
Text Solution
|
- Which of the following is an example of homogeneous catalytic reaction...
Text Solution
|
- The major product of the following reaction is :
Text Solution
|
- The type of hybridisation and number of lone pair(s) of electrons of X...
Text Solution
|
- Castner kellner process is given by:
Text Solution
|
- The major product formed in the reaction given below will be:
Text Solution
|
- Consider the following reduction processes : Zn^(2+)+2e^(-)toZn(s), ...
Text Solution
|
- The reducing power of the metal decreases in the order:
Text Solution
|
- Liquids A and B form an ideal solution in the entire composition range...
Text Solution
|
- A process has Delta H = 8 K J mol^(-1) " and " Delta S = 80JK^(-1) mo...
Text Solution
|
- The total number of isomers for a square planer complex [M(F)(Cl)(SCN)...
Text Solution
|
- Solution of CH3 COOH [Ka = 10^(-5) " at " 25^@ ] is been neutraliz...
Text Solution
|
- Number of peroxide bonds in the compound Cr2 O(9)^(2-) .
Text Solution
|