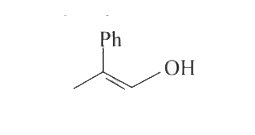
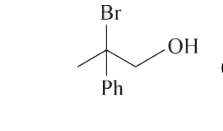
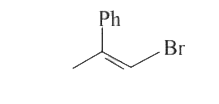
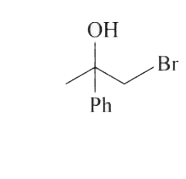
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-JEE MAIN REVISION TEST- 24 -CHEMISTRY (SECTION - 2)
- 1-methyl-1-phenyl ethylene oxide when treated with HBr produce
Text Solution
|
- Number of sp2 hybrid carbon atoms in aspartame is
Text Solution
|
- 6 gm of CH(3)COOH, 6 gm of NaOH and 6.3 gm of HNO(3) are dissolved in ...
Text Solution
|
- The radioisotpe, N-13,has a half-life of 10.0 minutes is used to imag...
Text Solution
|
- For the reaction 2A (g) + B(g) to C (g) Delta U^(@) = 20 kcal/mole, ...
Text Solution
|
- A gas 'X' is passed through water to form a saturated solution. The aq...
Text Solution
|