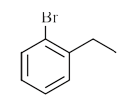
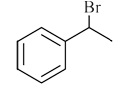
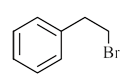
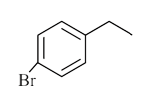
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-JEE MAIN REVISION TEST - 29 (2020)-CHEMISTRY
- Match the following :
Text Solution
|
- The IUPAC name of the complex [Co((SO4)(NH3)4]NO3 is
Text Solution
|
- Styrene when treated with HBr produces :
Text Solution
|
- A mixture of phenol and aromatic carboxylic acid can be separated easi...
Text Solution
|
- The number of orbitals associated with quantum numbers n =4 and m (8) ...
Text Solution
|
- Oxidation number of oxygen in K2O,K2O2 and KO2 respectively is :
Text Solution
|
Text Solution
|
- The atomic radius of Ru is closet to
Text Solution
|
- When zeolite which is hydrated sodium aluminium silicate is treated wi...
Text Solution
|
- Ellingham diagram represents change of
Text Solution
|
- The dipole moments of NH3, NF3 and H2O are in the order.
Text Solution
|
- The electron gain enthalpy (in kJ/mole) of F, O, N and C respectively ...
Text Solution
|
- What is the final product of following reaction ? Isopropyl alcohol ...
Text Solution
|
- The weakest inter molecular forces are present in:
Text Solution
|
- Consider the following reaction : The product 'X' is used :
Text Solution
|
- The increasing order of pKb for the following compounds will be :
Text Solution
|
- In which of the following coordination entities the magnitude of Delta...
Text Solution
|
- The number of oxygen atom in paracetamol is …..
Text Solution
|
- Two solution A and B, each of 80L was made by dissolving 4 gm NaOH and...
Text Solution
|
- Two radioactive nuclides A and B have half-lives 50 min and 10 min res...
Text Solution
|