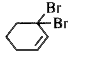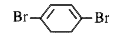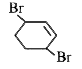A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-JEE MAIN REVISION TEST - 30 | JEE -2020-CHEMISTRY
- 1, 1-dimethyl ethylene oxide when treated with water in presence of ac...
Text Solution
|
- Among the following the one that gives positive iodoform test upon rea...
Text Solution
|
- The shortest wavelength spectral line in the brackett series of the sp...
Text Solution
|
- Oxidation number of Cr in K 2 Cr 2 O 7, CrO 5 and Cr 2 (SO 4 ...
Text Solution
|
- When 1-butyne undergoes oxymercuraction with the help of HgSO(4)+H(2)S...
Text Solution
|
- Atomic radius of Fe is closest to
Text Solution
|
- Ti and Zr are purified by
Text Solution
|
- Zeolite or permutit has the formula
Text Solution
|
- The dipolemoment of are in order
Text Solution
|
- The correct order of electron gain enthalpy (in kJ/mole) is
Text Solution
|
- What is the final product of the following reaction sequence? cyclo...
Text Solution
|
- The relative strength of intermolecular forces in bond decreasing orde...
Text Solution
|
- What is Hinsberg reagent?
Text Solution
|
- The decreasing order of Acidity for the following compound will be
Text Solution
|
- Which of the following does not have a metal-carbon bond?
Text Solution
|
- The number of chiral carbon in testosterone hormone?
Text Solution
|
- 100 mL of 0.1 M Na2 CO 3 solution is titrated with 0.1 M ...
Text Solution
|
- A first order reaction takes 69.3 minutes for 50% completion. How much...
Text Solution
|
- Work done during isothermal expansion of one mole of an ideal gas form...
Text Solution
|
- Write chemical equation for thermal decomposition of ammonium dichroma...
Text Solution
|