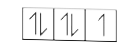A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-ATOMIC STRUCTURE-JEE Advanced (Archive )
- The triad of nuclei that is isotonic is:
Text Solution
|
- The wavelngth fo a spectrl line for an electronic transition is invers...
Text Solution
|
- The correct set of quantum numbers for the unpaired electron of chlori...
Text Solution
|
- The correct ground state electronic configuration of chromium atom is ...
Text Solution
|
- Which of the following does not characterise X-rays?
Text Solution
|
- Wave functions of electrons in atoms and molecules are called …………..
Text Solution
|
- The 2px and 2py, 2pz, orbitals of an atom have identical shapes but di...
Text Solution
|
- In a given electric field , the beta -particles are deflected more tha...
Text Solution
|
- The outermost electronic configuration of Cr .
Text Solution
|
- A 3p-orbital has :
Text Solution
|
- Which of the following relates to photon both as wave motion and as a...
Text Solution
|
- The orbital angular momentum of an electron in 3s-orbital is :
Text Solution
|
- Which of the following has the maximum number of unpaired electrons?
Text Solution
|
- For a d-electron, the orbital angular momentum is :
Text Solution
|
- The first use of quantam theroy to explain the structure of atom was m...
Text Solution
|
- The uncertainty principle and the concept of wave nature of matter wer...
Text Solution
|
- Which of the following statement (s) is (are) correct?
Text Solution
|
- Ground state electronic configuration of nitrogen atom can be represen...
Text Solution
|
- Assertion: The first ionisation energy of Be is greater than that of B...
Text Solution
|
- The number of nodal planes in a p(x) orbital is :
Text Solution
|