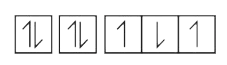
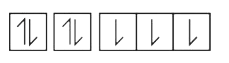
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-ATOMIC STRUCTURE-JEE Advanced (Archive )
- The uncertainty principle and the concept of wave nature of matter wer...
Text Solution
|
- Which of the following statement (s) is (are) correct?
Text Solution
|
- Ground state electronic configuration of nitrogen atom can be represen...
Text Solution
|
- Assertion: The first ionisation energy of Be is greater than that of B...
Text Solution
|
- The number of nodal planes in a p(x) orbital is :
Text Solution
|
- The electronic configuration of an element is 1s^2 2s^2 2p^6 3s^2 3p^6...
Text Solution
|
- The wavelength associated with a golf ball weighing 200 g and moving a...
Text Solution
|
- The quantum number + 1//2 and -1//2 for the electron spin represent
Text Solution
|
- Rutherfords experiments , which established the nuclear model of atom ...
Text Solution
|
- If the nitrogen atom has electronic configuration 1s^(7), it would hav...
Text Solution
|
- The radius of which of the following orbit is the same as that of the ...
Text Solution
|
- The numbers of radial nodes of 3s and 2p orbitals are respectively:
Text Solution
|
- According to Bohr’s theory E(n)= Total energy, K(n)= Kinetic energy...
Text Solution
|
- Match the entries in column I with the correctly related quantum numbe...
Text Solution
|
- The hydrogen -like species Li^(2+) is in a spherically symmetric stat...
Text Solution
|
- The hydrogen-like species Li^(2+) is in a spherically symmetric stat...
Text Solution
|
- The hydrogen-like species Li^(2+) is in a spherically symmetric stat...
Text Solution
|
- The kinetic energy of an electron in the second Bohr orbit of a hydrog...
Text Solution
|
- P is the probability of finding the 1s electron of hydrogen atom in a ...
Text Solution
|
- The wave function, Psi("n, l, m(l)) is a mathematical function whose...
Text Solution
|