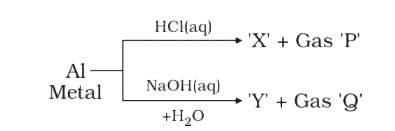
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
p-BLOCK ELEMENTS-1
VMC MODULES ENGLISH|Exercise JEE Main Archive|34 Videosp-BLOCK ELEMENTS-1
VMC MODULES ENGLISH|Exercise JEE Advanced Archive|48 Videosp-BLOCK ELEMENTS-1
VMC MODULES ENGLISH|Exercise LEVEL 1|75 VideosMOCK TEST 9
VMC MODULES ENGLISH|Exercise CHEMISTRY (SECTION 2)|5 VideosPERIODIC PROPERTIES OF ELEMENTS
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-p-BLOCK ELEMENTS-1-LEVEL 2
- Identify the correct resonance structures of carbon dioxide from the o...
Text Solution
|
- Silicon dissolves in excess of HF due to formation of
Text Solution
|
- The incorrect statement regarding below reaction is :
Text Solution
|
- The plague OR tin pest or tin disease refers to .
Text Solution
|
- H(2)C(2)O(4)overset(triangle)to"gas"(A)+"gas"(B)+"liquid"(C). Gas(A) b...
Text Solution
|
- Amphilbole silicate structure has 'x' number of corner shared per tetr...
Text Solution
|
- The silicate ion in the mineral kinoite is a chain of three SiO(4)^(4-...
Text Solution
|
- BX(3)+NH(3) overset(B.T.) to BX(3) *NH(3)+Heat of adduct formation (De...
Text Solution
|
- Statement-1: Al(OH)(3) is amphoteric in nature. Statement-2: It cann...
Text Solution
|
- Assertion : Among SiCl4 and C Cl4 only SiCl4 reacts with water. R...
Text Solution
|
- Statement I: Pb^(4+) compounds are stronger oxidizing agents than Sn^(...
Text Solution
|
- Consider of following reactions CHF(3) overset(K(a))(to)CF(3)^(-)+H^...
Text Solution
|
- Choose the correct order of C-C bond length in the given compounds:
Text Solution
|
- The correct order of thermal stability of silicon tetrahalides is
Text Solution
|
- Which of the following statements is true ?
Text Solution
|
- Which of the following process is/are assciated with change of hybridi...
Text Solution
|
- Which of the following is/are correct statement ?
Text Solution
|
- Which of the following statements is/are correct
Text Solution
|
- Choose the incorrect statement (s) from the following
Text Solution
|
- SiO(2) reacts with
Text Solution
|