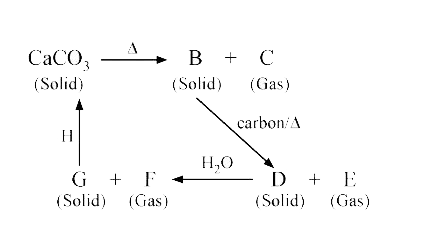
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
p-BLOCK ELEMENTS-1
VMC MODULES ENGLISH|Exercise JEE Main Archive|34 Videosp-BLOCK ELEMENTS-1
VMC MODULES ENGLISH|Exercise JEE Advanced Archive|48 Videosp-BLOCK ELEMENTS-1
VMC MODULES ENGLISH|Exercise LEVEL 1|75 VideosMOCK TEST 9
VMC MODULES ENGLISH|Exercise CHEMISTRY (SECTION 2)|5 VideosPERIODIC PROPERTIES OF ELEMENTS
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-p-BLOCK ELEMENTS-1-LEVEL 2
- The compound E is
Text Solution
|
- The correct statement about F is
Text Solution
|
- The compound H is :
Text Solution
|
- total number of molecule which hydrolysed at room temperature and hybr...
Text Solution
|
- The difference between total number of lone pairs and total number of ...
Text Solution
|
- Borazine is converted into a disubstituted product B(3)N(3)H(4)X(2)....
Text Solution
|
- Consider the structure of Al(2)Me(6) compound and find the value of (x...
Text Solution
|
- Find the value of x in the tremolite abestos: Ca(2)Mg(x)(Si(4)O(11)...
Text Solution
|
- Consider the following silicates (a) BaTi(Si(2)O(9)) (b) ZnCa(2)Si...
Text Solution
|
- Consider Al(2)(OH)(6) compound and calculate the vale of (X+Y):=Z Wh...
Text Solution
|
- Number of hydroxyl groups present in H(4)P(2)O(6) are :
Text Solution
|
- Write down the electronic configuration of: ltBrgt (i) Cr^(3+) (ii)...
Text Solution
|
- Consider the following species: (C(3)H(5))(3)Al Find out total numbe...
Text Solution
|
- Consider the following species: (i) CH(3)^(+) (ii) (C(3)H(5))(3)Al...
Text Solution
|
- Consider the following species: (i) CH(3)^(+) (ii) (C(3)H(5))(3)Al...
Text Solution
|
- Consider the following species and find out total number of species wh...
Text Solution
|
- Consider the following species: (i) CH(3)^(+) (ii) (C(3)H(5))(3)Al...
Text Solution
|
- Consider the following species: (i) CH(3)^(+) (ii) (C(3)H(5))(3)Al...
Text Solution
|
- Consider the following species: (i) CH(3)^(+) (ii) (C(3)H(5))(3)Al...
Text Solution
|
- Consider the following species: (i) CH(3)^(+) (ii) (C(3)H(5))(3)Al...
Text Solution
|