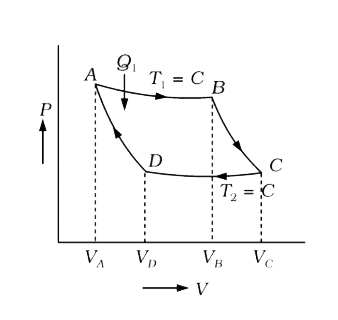
Text Solution
Verified by Experts
Topper's Solved these Questions
GASEOUS STATE & THERMODYNAMICS
VMC MODULES ENGLISH|Exercise Level - 1|75 VideosGASEOUS STATE & THERMODYNAMICS
VMC MODULES ENGLISH|Exercise Level - 2|40 VideosGASEOUS STATE & THERMODYNAMICS
VMC MODULES ENGLISH|Exercise level-0 Short Answer Type – I|10 VideosENERGY & MOMENTUM
VMC MODULES ENGLISH|Exercise JEE ADVANCE (ARCHIVE) - TRUE/FALSE TYPE|1 VideosGRAVITATION
VMC MODULES ENGLISH|Exercise JEE Advance (Archive) TRUE/FALSE|1 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-GASEOUS STATE & THERMODYNAMICS-level-0 Short Answer Type – II
- Statement-1 : The third law of thermodynamics implies that absolute ze...
Text Solution
|
- In changing the state of a gas adiabatically from an equilibrium state...
Text Solution
|
- A gas expands in such a manner that its pressure and volume comply wit...
Text Solution
|
- Show that the slope of adiabatic curve at any point is Yama times the...
Text Solution
|
- The volume V versus temperature T graphs for a certain amount of a per...
Text Solution
|
- Define Cp and CV. Why is CP gt CV ? For an ideal gas, prove that CP - ...
Text Solution
|
- Two gases have the same initial pressure P, volume V and temperature T...
Text Solution
|
- Consider two containers A and B containing identical gases at the same...
Text Solution
|
- Two gases have the same initial pressure P, volume V and temperature T...
Text Solution
|
- Cooling is produced when a gas at high pressure suddenly expands. Why?
Text Solution
|
- When a gas is suddenly compressed, its temperature rises. Why?
Text Solution
|
- Is it possible in increase the temperature of a gas without adding hea...
Text Solution
|
- Can the temperature of a gas increased keeping its pressure and volume...
Text Solution
|
- Two carnot engines A and B are operated in series. The first one A rec...
Text Solution
|
- Two carnot engines A and B are operated in series. The first one A rec...
Text Solution
|
- During a process, the volume of a gas is found to depend inversely on ...
Text Solution
|
- Can two isothermal curves intersect each other?
Text Solution
|
- What is an isothermal process? State essential conditions for such a p...
Text Solution
|
- Define an adiabatic process and state essential conditions for such a ...
Text Solution
|
- Carnot engine is
Text Solution
|