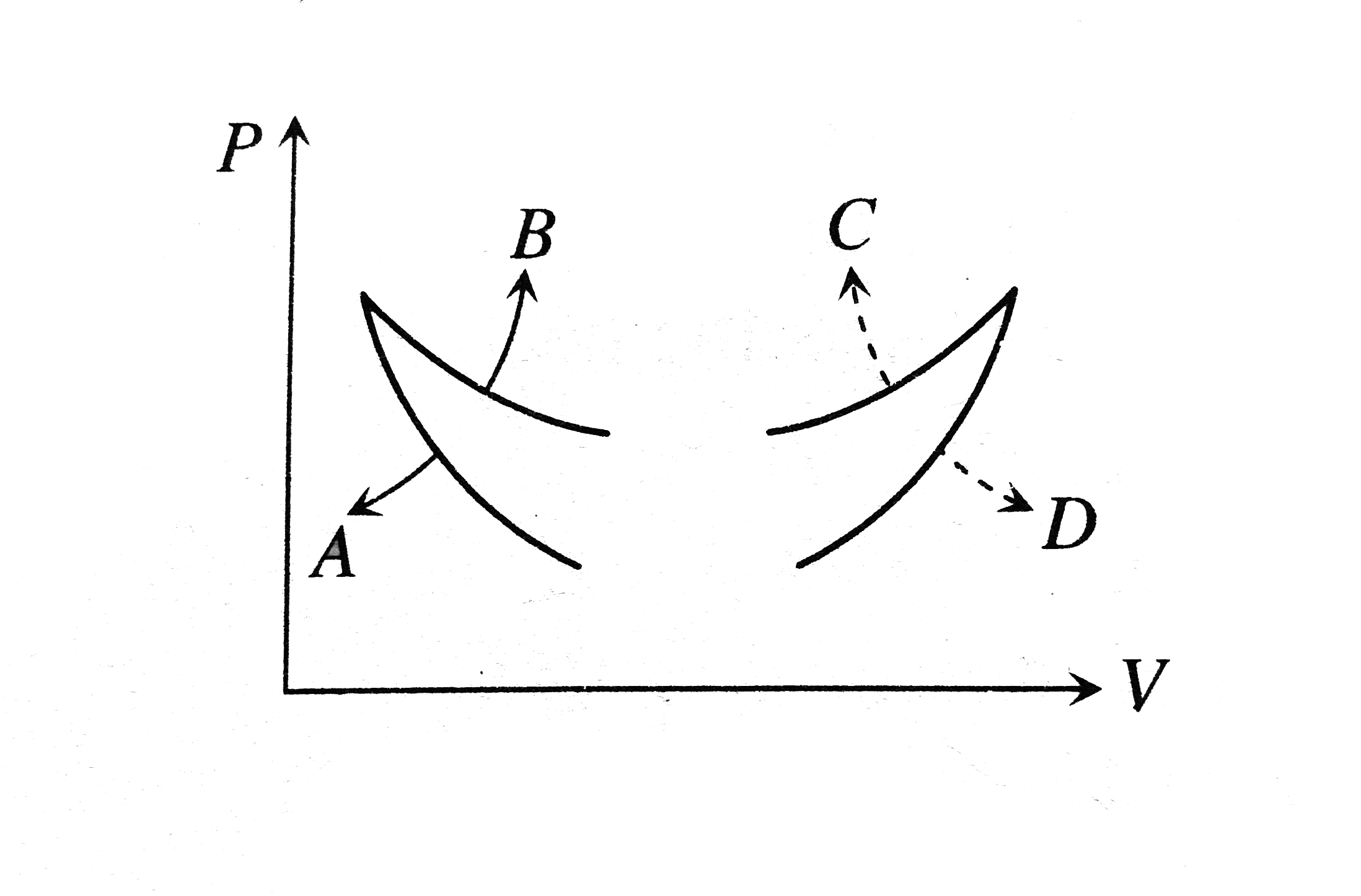
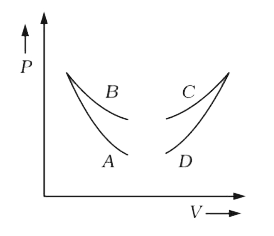
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GASEOUS STATE & THERMODYNAMICS
VMC MODULES ENGLISH|Exercise Level - 2|40 VideosGASEOUS STATE & THERMODYNAMICS
VMC MODULES ENGLISH|Exercise JEE MAIN (ARCHIVE )|81 VideosGASEOUS STATE & THERMODYNAMICS
VMC MODULES ENGLISH|Exercise level-0 Short Answer Type – II|24 VideosENERGY & MOMENTUM
VMC MODULES ENGLISH|Exercise JEE ADVANCE (ARCHIVE) - TRUE/FALSE TYPE|1 VideosGRAVITATION
VMC MODULES ENGLISH|Exercise JEE Advance (Archive) TRUE/FALSE|1 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-GASEOUS STATE & THERMODYNAMICS-Level - 1
- The average degree of freedom per molecule for a gas is 6. The gas per...
Text Solution
|
- 30 litre of a gas at STP is isothermally compressed to 6 litre. Amount...
Text Solution
|
- Four curves A, B, C and D are drawn in Fig. for a given amount of gas....
Text Solution
|
- Two adiabatic expansions of n mole of same gas are shown. If VB/VA = V...
Text Solution
|
- Ideal mono-atomic gas is taken through process such that dQ = 3dU. The...
Text Solution
|
- When an ideal gas at pressure P, temperature T and volume V is isother...
Text Solution
|
- During adiabatic process, pressure P and density equation is:
Text Solution
|
- For a gas of molecular weight M specific heat capacity at constatn pre...
Text Solution
|
- p-V diagram of a diatomic gas is a straight line passing through origi...
Text Solution
|
- One mole of an ideal gas (mono-atomic) at temperature T0 expands slow...
Text Solution
|
- A mono-atomic gas is taken along path AB as shown. Calculate change in...
Text Solution
|
- A gas is compressed adiabatically till its pressure becomes 27 times i...
Text Solution
|
- The volume of one mole of ideal gas with adiabatic exponent is varie...
Text Solution
|
- In a cyclic process shown in the figure an ideal gas is adiabatically ...
Text Solution
|
- A gas is taken through a cyclic process ABCA as shown in, if 2.4 cal o...
Text Solution
|
- A carnot engine has the same efficiency (i) between 100 K and 500 K an...
Text Solution
|
- If a system undergoes an adiabatic change from state 1 to state 2, the...
Text Solution
|
- Two different ideal diatomic gases A and B are initially in the same s...
Text Solution
|
- A Carnot refrigerator works between two temperatures of 300 K & 600 K....
Text Solution
|
- In the following P-V diagram two adiabatics cut two isothermals at tem...
Text Solution
|