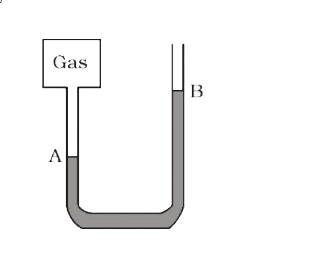
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GASEOUS STATE & THERMODYNAMICS
VMC MODULES ENGLISH|Exercise Level - 2|40 VideosGASEOUS STATE & THERMODYNAMICS
VMC MODULES ENGLISH|Exercise JEE MAIN (ARCHIVE )|81 VideosGASEOUS STATE & THERMODYNAMICS
VMC MODULES ENGLISH|Exercise level-0 Short Answer Type – II|24 VideosENERGY & MOMENTUM
VMC MODULES ENGLISH|Exercise JEE ADVANCE (ARCHIVE) - TRUE/FALSE TYPE|1 VideosGRAVITATION
VMC MODULES ENGLISH|Exercise JEE Advance (Archive) TRUE/FALSE|1 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-GASEOUS STATE & THERMODYNAMICS-Level - 1
- An ideal gas with adiabatic exponent gamma = 4/3 undergoes a process ...
Text Solution
|
- Three moles of an ideal monoatomic gas perform a cycle shown in figure...
Text Solution
|
- A Carnot engine takes 3xx10^6cal. of heat from a reservoir at 627^@C, ...
Text Solution
|
- An ideal Carnot engine, whose efficiency is 40% receives heat at 500 K...
Text Solution
|
- Figure shows the variation of internal energy (U) with the pressure (P...
Text Solution
|
- A sample of gas is allowed to expand adiabatically. As a consequence i...
Text Solution
|
- A heat engine with a thermal efficiency of 40% does 100 J of work per ...
Text Solution
|
- Figure shows an ideal gas. Its pressure, volume and temperature are P0...
Text Solution
|
- Figure shows a container having adiabatic walls and a freely movable s...
Text Solution
|
- Three moles of an diatomic gas are in a closed rigid container at temp...
Text Solution
|
- Calculate the pressure (in N/m^2)exerted by a mixture of 8 g of oxygen...
Text Solution
|
- A reversible engine takes heat from a reservoir at 527^(@)C and gives ...
Text Solution
|
- If the P-V diagram of a diatomic gas is plotted, it is a straight line...
Text Solution
|
- A heat of 1200 J is supplied to an engine from a hot reservoir maintai...
Text Solution
|
- If the work done by a Carnot engine working between two temperatures 6...
Text Solution
|
- A vessel A of volume 3V contains a gas at pressure 4p(0) and a vessel ...
Text Solution
|
- A vessel of volume 0.2 m^(3) contains hydrogen gas at temperature 300 ...
Text Solution
|
- A Vessel contains helium, which expands at constant pressure when 15 k...
Text Solution
|
- 4 mole of an ideal gas at 27^@ C is isothermally expanded to 7 times i...
Text Solution
|
- One mole of a monatomic gas is taken from a point A to another point B...
Text Solution
|