A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-GASEOUS STATE & THERMODYNAMICS-JEE ADVANCED (ARCHIVE )
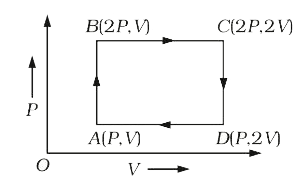
- An ideal gas is taken around the cycle ABCDA as shown in the P-V diagr...
Text Solution
|
- At room temperature, the rms speed of the molecules of a certain diato...
Text Solution
|
- 70 calories of heat is required to raise the temperature of 2 mole of ...
Text Solution
|
- If one mole of a mono-atomic gas (gamma=5//3) is mixed with one mole o...
Text Solution
|
- When an ideal diatomic gas is heated at constant pressure the fraction...
Text Solution
|
- Three closed vessels (A),(B) and C are at the same temperature (T) and...
Text Solution
|
- From the following statements concerning ideal gas at any given temper...
Text Solution
|
- The temperature of an ideal gas is increased from 120 K to 480 K. If a...
Text Solution
|
- A ring shaped tube contain two ideal gases with equal masses and mola...
Text Solution
|
- The average translational energy and the rms speed of molecules in a s...
Text Solution
|
- The average translational kinetic energy of O(2) (molar mass 32) molec...
Text Solution
|
- A vessel contains 1 mole of O(2) gas (molar mass 32) at a temperature ...
Text Solution
|
- A vessel contains a mixture of one mole of Oxygen and two moles of Nit...
Text Solution
|
- Two identical cylinders A and B with frictionless pistons contain the ...
Text Solution
|
- Two cylinders fitted with pistons contain equal amount of an ideal dia...
Text Solution
|
- A given quantity of a ideal gas is at pressure P and absolute tempera...
Text Solution
|
- A gas mixture coinsists of (2) moles of oxygen and (4) moles of argon ...
Text Solution
|
- Starting with the same initial conditions, an ideal gas expands from v...
Text Solution
|
- An ideal gas is initially at temperature T and volume V. ITS volume is...
Text Solution
|
- In a given process on an ideal gas, dW = 0 and dQ lt 0. Then for the g...
Text Solution
|