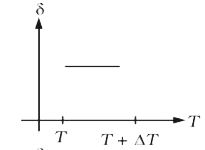
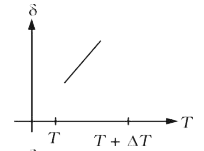
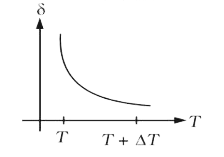
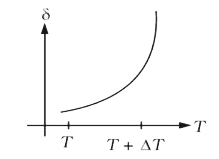
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-GASEOUS STATE & THERMODYNAMICS-JEE ADVANCED (ARCHIVE )
- A gas mixture coinsists of (2) moles of oxygen and (4) moles of argon ...
Text Solution
|
- Starting with the same initial conditions, an ideal gas expands from v...
Text Solution
|
- An ideal gas is initially at temperature T and volume V. ITS volume is...
Text Solution
|
- In a given process on an ideal gas, dW = 0 and dQ lt 0. Then for the g...
Text Solution
|
- p-V plots for two gases during adiabatic process as shown in figure pl...
Text Solution
|
- Which of the following graphs correctly represents the variation of be...
Text Solution
|
- A monoatomic ideal gas, initially at temperature T1, is enclosed in a ...
Text Solution
|
- An ideal gas is taken through cycle A to B to C-A, as shown in Fig. IF...
Text Solution
|
- An ideal gas undergoes a cyclic process as shown in the given P-T diag...
Text Solution
|
- An ideal gas is initially at P1,V1 is expands isothermally to P2,V2 an...
Text Solution
|
- The presssure of a medium is changed from 1.01xx10^(5) Pa to 1.165xx...
Text Solution
|
- An ideal gas is expanding such that PT^2= constant. The coefficient of...
Text Solution
|
- A real gas behaves like an ideal gas if its
Text Solution
|
- 5.6 litre of helium gas at STP is adiabatically compressed to 0.7 litr...
Text Solution
|
- A mixture of 2 moles of helium gas ( (atomic mass)=4a.m.u ) and 1 mole...
Text Solution
|
- Two moles of ideal helium gas are in a rubber balloon at 30^@C. The ba...
Text Solution
|
- Two non-reactive monoatomic ideal gases have their atomic masses in th...
Text Solution
|
- A gas is enclosed in a cylinder with a movable frictionless piston. It...
Text Solution
|
- For an ideal gas
Text Solution
|
- An ideal gas is taken from the state A (pressure p, volume V) to the s...
Text Solution
|