Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-GASEOUS STATE & THERMODYNAMICS-JEE ADVANCED (ARCHIVE )
- Two glass bulbs of equal volume are connected by a narrow tube and are...
Text Solution
|
- A thin tube of uniform cross-section is sealed at both ends. It lies h...
Text Solution
|
- The pressure at C is
Text Solution
|
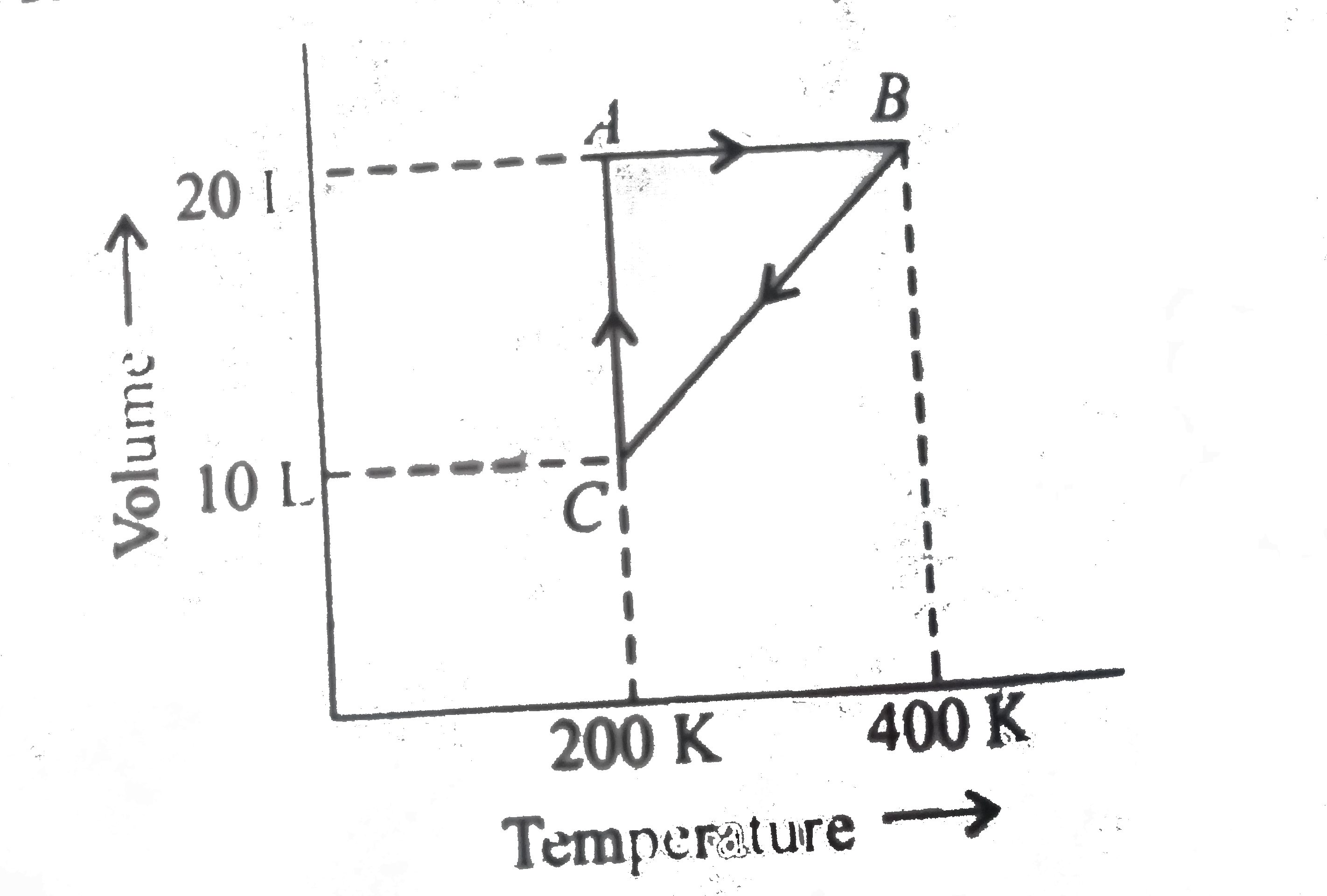
- One mole of a monatomic ideal gas is taken through the cycle shown in ...
Text Solution
|
- One mole of a monatomic ideal gas is taken through the cycle shown in ...
Text Solution
|
- An ideal gas is taken through a cyclic thermodynamic process through f...
Text Solution
|
- An ideal gas is taken through a cyclic thermodynamic process through f...
Text Solution
|
- At 27^(@)C two moles of an ideal monatomic gas occupy a volume. V The...
Text Solution
|
- At 27^(@)C two moles of an ideal monatomic gas occupy a volume. V The...
Text Solution
|
- At 27^(@)C two moles of an ideal monatomic gas occupy a volume. V The...
Text Solution
|
- One mole of a diatomic ideal gas (gamma=1.4) is taken through a cyclic...
Text Solution
|
- One mole of a diatomic ideal gas (gamma = 1.4) is taken through a cyc...
Text Solution
|
- One mole of a diatomic ideal gas (gamma = 1.4) is taken through a cyc...
Text Solution
|
- A sample of 2 kg monoatomic helium (assumed ideal ) is taken from A to...
Text Solution
|
- A sample of 2 kg monoatomic helium (assumed ideal) is taken through t...
Text Solution
|
- A mono-atomic ideal gas of two moles is taken through a cyclic process...
Text Solution
|
- A monoatomic ideal gas of two moles is taken through a cyclic process ...
Text Solution
|
- The piston cylinder arrangement shown contains a diatomic gas at tempe...
Text Solution
|
- A diatomic gas is enclosed in a vessel fitted with massless movable pi...
Text Solution
|
- A diatomic ideal gas is compressed adiabatically to 1/32 of its initia...
Text Solution
|