Text Solution
Verified by Experts
The correct Answer is:
VMC MODULES ENGLISH-GASEOUS STATE & THERMODYNAMICS-JEE ADVANCED (ARCHIVE )
- A monoatomic ideal gas of two moles is taken through a cyclic process ...
Text Solution
|
- The piston cylinder arrangement shown contains a diatomic gas at tempe...
Text Solution
|
- A diatomic gas is enclosed in a vessel fitted with massless movable pi...
Text Solution
|
- A diatomic ideal gas is compressed adiabatically to 1/32 of its initia...
Text Solution
|
- A thermodynamic system is taken from an initial state I with internal ...
Text Solution
|
- One mole of a monatomic ideal gas undergoes an adiabatic expansion in ...
Text Solution
|
- "A solid sphere of copper of radius R and a hollow sphere of the same ...
Text Solution
|
- One mole of a monatomic ideal gas is mixed with one mole of a diatomic...
Text Solution
|
- During an experiment, an ideal gas is found to obey an additional law ...
Text Solution
|
- An ideal gas has a molar heat capacity at constant pressure of Cp = 2....
Text Solution
|
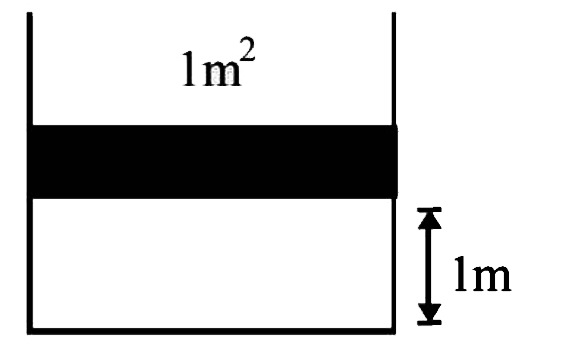
- A container of volume 1m^3 is divided into two equal parts by a partit...
Text Solution
|
- An ideal gas with pressure P, volume V and temperature T is expanded i...
Text Solution
|
- A closed container of volume 0.02m^3contains a mixture of neon and arg...
Text Solution
|
- A gas thermometer is used as a standard thermometer for measurement of...
Text Solution
|
- One mole of an ideal mono-atomic gas is taken round cyclic process ABC...
Text Solution
|
- One mole of ideal monoatomic gas is taken round the cyclic process a...
Text Solution
|
- One mole of an ideal mono-atomic gas is taken round cyclic process ABC...
Text Solution
|
- One mole of an ideal mono-atomic gas is taken round cyclic process ABC...
Text Solution
|
- An insulated box containing a monoatomic gas of molar mass (M) moving ...
Text Solution
|
- The volume versus temperature graph for a certain amount of a perfect ...
Text Solution
|