Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise Level - 0( Short Answer Type-II)|8 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise Level - 0 (Long Answer Type)|4 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-L|9 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE - G|10 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL BONDING-I & II-JEE Advanced (Archive)
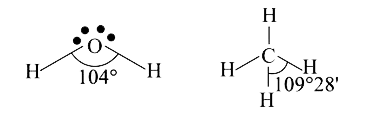
- Why is the bond angle of water less than the bond angle of CH(4)?
Text Solution
|
- State four major physical properties than can be used to distingusish ...
Text Solution
|
- The compound which contains both ionic and covalent bonds is
Text Solution
|
- Element X is strongly electropositive and Y is strongly electronegativ...
Text Solution
|
- Which of the following compounds is covalent ? .
Text Solution
|
- If a molecule MX(3) has zero dipole moment , the sigma bonding orbital...
Text Solution
|
- Pair of molecules which forms strongest intermolecular hydrogen bonds ...
Text Solution
|
- The angle between two covalent bonds is maximum for (CH(4), H(2)O, CO(...
Text Solution
|
- …………… hybrid orbitals of nitrogen atom are involved in the formation o...
Text Solution
|
- The ion that is isoelectronic with CO is
Text Solution
|
- Among the following, the linear molecule is
Text Solution
|
- There are …………. pi bonds in a nitrogen molecule
Text Solution
|
- Which one among the following does not have the hybrogen bond ?
Text Solution
|
- Write the Lewis dot structural formula for each of the following. Give...
Text Solution
|
- Carbon tetrachloride has no net dipole momnet because of .
Text Solution
|
- Linear overlap of two atomic p-orbitals leads to a sigma bond .
Text Solution
|
- On hydridisation of one s and one p-orbitals , we get
Text Solution
|
- SnCI(2) is a non-linear molecule .
Text Solution
|
- The hybridisaton of sulphur in sulphur dioxide is .
Text Solution
|
- CO(2) is isostructural with
Text Solution
|
- The bond between two indentical non-metal atoms has a pair of electron...
Text Solution
|