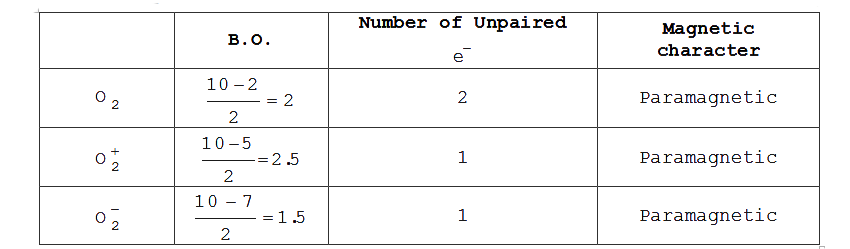Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise Level - 0 (Long Answer Type)|4 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise Level - 1 (JEE Main)|75 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|98 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-L|9 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE - G|10 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL BONDING-I & II-Level - 0( Short Answer Type-II)
- Define bond length and bond energy and state the relationship between ...
Text Solution
|
- Arrange the F(2), F(2)^(+) and F(2)^(-) in increasing order of bond...
Text Solution
|
- Using molelcular orbital theory, compare the bond energy and magnetic ...
Text Solution
|
- The bond dissociation energy of N(2)^(+) is less than that of N(2) ....
Text Solution
|
- Is it possible for Ne(2) and F(2)^(-) to exist?
Text Solution
|
- Calculate the formal charge on O atoms numbered as 1 , 2 and 3 of O(3...
Text Solution
|
- Calculate the percent ionic character of HCl. Given that the observed ...
Text Solution
|
- Arrange the following in order of decreasing bond angles (i) CH(4), ...
Text Solution
|