Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Main (Archive)|62 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|98 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise Level - 2 (JEE Advanced)|50 VideosCHEMICAL BONDING & MOLECULAR STRUCTURE
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-L|9 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE - G|10 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL BONDING-I & II-JEE Main (Numerical Value Questions)
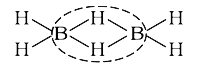
- The number of three centre two electron bonds in a molecule of diboran...
Text Solution
|
- How many hydrogen bonded water molecules are associated with CuSO(4). ...
Text Solution
|
- How many equitorial bonds are there are in PCl(5) ?
Text Solution
|
- The number of completely filled pi molecular orbitals in ground state ...
Text Solution
|
- The number of non-bonding electrons of N(2) is.
Text Solution
|
- The pi^(**) molecular orbital has nodal planes.
Text Solution
|
- How many lone pairs are there at xenon in XeOF(4) ?
Text Solution
|
- Number of p pi-d pi bonds present in XeO(4) are
Text Solution
|
- The tota number of P-O bonds in P(4)O(10) is
Text Solution
|
- Total number of covalent bonds in C(3)O(2) is The total number of si...
Text Solution
|
- The maximum possible number of hydrogen bonds in a water molecule can ...
Text Solution
|
- How many 'sp' hybrid orbital are there in allene C(3)H(4) ?
Text Solution
|
- Total number of species among following, in which bond angle is equal...
Text Solution
|
- Total number of species that can be oxidzed by acidic permanganate ion...
Text Solution
|
- Total number of molecules which can form H-bond among themselves. Si...
Text Solution
|